Null anticarcinogenic effect of silymarin on diethylnitrosamine-induced hepatocarcinogenesis in rats
- PMID: 24348760
- PMCID: PMC3861306
- DOI: 10.3892/etm.2013.1391
Null anticarcinogenic effect of silymarin on diethylnitrosamine-induced hepatocarcinogenesis in rats
Abstract
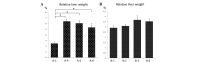
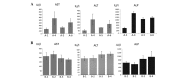
The aim of this study was to investigate the anticarcinogenic effects of silymarin in diethylnitrosamine (DEN)-induced hepatocarcinogenic rat models. Severe and mild models of hepatocellular carcinoma (HCC) were generated by the intraperitoneal administration of 40 mg/kg DEN once a week for 18 weeks and 100 mg/kg DEN every 2 weeks for 6 weeks in male Wistar rats, respectively. In the severe and mild models of HCC, the rats were treated with 0.1 and 0.5% silymarin for 18 weeks and with 0.1% silymarin for 5 weeks, respectively. Serum transaminase levels were not significantly decreased by the silymarin treatment in either model. Macroscopic and microscopic features indicated that the silymarin-containing formulations did not significantly inhibit the hepatic tumor formation induced by DEN. Furthermore, immunohistochemical and western blot analyses demonstrated that the expression levels of proliferating cell nuclear antigen and glutathione S-transferase P, which are hepatocarcinogenic markers, were not significantly modified by the silymarin treatment. These results indicate that silymarin may not be considered as a candidate agent against hepatocarcinogenesis.
Keywords: diethylnitrosamine; hepatocellular carcinoma; silymarin.
Figures







References
-
- Bosch FX, Ribes J, Borràs J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–285. - PubMed
-
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. - PubMed
-
- Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. - PubMed
-
- Makuuchi M, Kokudo N, Arii S, et al. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res. 2008;38:37–51. - PubMed
-
- Avila MA, Berasain C, Sangro B, Prieto J. New therapies for hepatocellular carcinoma. Oncogene. 2006;25:3866–3884. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
