Risk of severe upper gastrointestinal complications among oral bisphosphonate users
- PMID: 24348985
- PMCID: PMC3857168
- DOI: 10.1371/journal.pone.0073159
Risk of severe upper gastrointestinal complications among oral bisphosphonate users
Abstract
Background: Oral bisphosphonates (BPs) are the primary agents for the treatment of osteoporosis. Although BPs are generally well tolerated, serious gastrointestinal adverse events have been observed.
Aim: To assess the risk of severe upper gastrointestinal complications (UGIC) among BP users by means of a large study based on a network of Italian healthcare utilization databases.
Methods: A nested case-control study was carried out by including 110,220 patients aged 45 years or older who, from 2003 until 2005, were treated with oral BPs. Cases were the 862 patients who experienced the outcome (hospitalization for UGIC) until 2007. Up to 20 controls were randomly selected for each case. Conditional logistic regression model was used to estimate odds ratio (OR) associated with current use of BPs after adjusting for several covariates. A set of sensitivity analyses was performed in order to account for sources of systematic uncertainty.
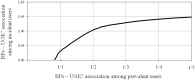
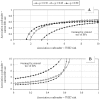
Results: The adjusted OR for current use of BPs with respect to past use was 0.94 (95% CI 0.81 to 1.08). There was no evidence that this risk changed either with BP type and regimen, or concurrent use of other drugs or previous hospitalizations.
Conclusions: No evidence was found that current use of BPs increases the risk of severe upper gastrointestinal complications compared to past use.
Conflict of interest statement
Figures


References
-
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285: 785–95. - PubMed
-
- Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, et al. (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 333: 1437–43. - PubMed
-
- Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, et al. (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348: 1535–41. - PubMed
-
- Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, et al. (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 280: 2077–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

