Nifedipine treatment reduces resting calcium concentration, oxidative and apoptotic gene expression, and improves muscle function in dystrophic mdx mice
- PMID: 24349043
- PMCID: PMC3857175
- DOI: 10.1371/journal.pone.0081222
Nifedipine treatment reduces resting calcium concentration, oxidative and apoptotic gene expression, and improves muscle function in dystrophic mdx mice
Abstract
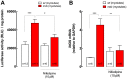
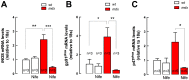
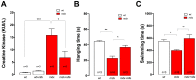
Duchenne Muscular Dystrophy (DMD) is a recessive X-linked genetic disease, caused by mutations in the gene encoding dystrophin. DMD is characterized in humans and in mdx mice by a severe and progressive destruction of muscle fibers, inflammation, oxidative/nitrosative stress, and cell death. In mdx muscle fibers, we have shown that basal ATP release is increased and that extracellular ATP stimulation is pro-apoptotic. In normal fibers, depolarization-induced ATP release is blocked by nifedipine, leading us to study the potential therapeutic effect of nifedipine in mdx muscles and its relation with extracellular ATP signaling. Acute exposure to nifedipine (10 µM) decreased [Ca(2+)]r, NF-κB activity and iNOS expression in mdx myotubes. In addition, 6-week-old mdx mice were treated with daily intraperitoneal injections of nifedipine, 1 mg/Kg for 1 week. This treatment lowered the [Ca(2+)]r measured in vivo in the mdx vastus lateralis. We demonstrated that extracellular ATP levels were higher in adult mdx flexor digitorum brevis (FDB) fibers and can be significantly reduced after 1 week of treatment with nifedipine. Interestingly, acute treatment of mdx FDB fibers with apyrase, an enzyme that completely degrades extracellular ATP to AMP, reduced [Ca(2+)]r to a similar extent as was seen in FDB fibers after 1-week of nifedipine treatment. Moreover, we demonstrated that nifedipine treatment reduced mRNA levels of pro-oxidative/nitrosative (iNOS and gp91(phox)/p47(phox) NOX2 subunits) and pro-apoptotic (Bax) genes in mdx diaphragm muscles and lowered serum creatine kinase (CK) levels. In addition, nifedipine treatment increased muscle strength assessed by the inverted grip-hanging test and exercise tolerance measured with forced swimming test in mdx mice. We hypothesize that nifedipine reduces basal ATP release, thereby decreasing purinergic receptor activation, which in turn reduces [Ca(2+)]r in mdx skeletal muscle cells. The results in this work open new perspectives towards possible targets for pharmacological approaches to treat DMD.
Conflict of interest statement
Figures






Similar articles
-
Increased resting intracellular calcium modulates NF-κB-dependent inducible nitric-oxide synthase gene expression in dystrophic mdx skeletal myotubes.J Biol Chem. 2012 Jun 15;287(25):20876-87. doi: 10.1074/jbc.M112.344929. Epub 2012 May 1. J Biol Chem. 2012. PMID: 22549782 Free PMC article.
-
Altered ROS production, NF-κB activation and interleukin-6 gene expression induced by electrical stimulation in dystrophic mdx skeletal muscle cells.Biochim Biophys Acta. 2015 Jul;1852(7):1410-9. doi: 10.1016/j.bbadis.2015.03.012. Epub 2015 Apr 7. Biochim Biophys Acta. 2015. PMID: 25857619 Free PMC article.
-
Effect of 2-Aminoethoxydiphenyl Borate on the State of Skeletal Muscles in Dystrophin-Deficient mdx Mice.Front Biosci (Landmark Ed). 2024 Dec 25;29(12):428. doi: 10.31083/j.fbl2912428. Front Biosci (Landmark Ed). 2024. PMID: 39735998
-
Pharmacological control of cellular calcium handling in dystrophic skeletal muscle.Neuromuscul Disord. 2002 Oct;12 Suppl 1:S155-61. doi: 10.1016/s0960-8966(02)00095-0. Neuromuscul Disord. 2002. PMID: 12206810 Review.
-
The Role of P2X7 Purinoceptors in the Pathogenesis and Treatment of Muscular Dystrophies.Int J Mol Sci. 2023 May 29;24(11):9434. doi: 10.3390/ijms24119434. Int J Mol Sci. 2023. PMID: 37298386 Free PMC article. Review.
Cited by
-
Reduction of Mitochondrial Calcium Overload via MKT077-Induced Inhibition of Glucose-Regulated Protein 75 Alleviates Skeletal Muscle Pathology in Dystrophin-Deficient mdx Mice.Int J Mol Sci. 2024 Sep 13;25(18):9892. doi: 10.3390/ijms25189892. Int J Mol Sci. 2024. PMID: 39337383 Free PMC article.
-
Whole body periodic acceleration is an effective therapy to ameliorate muscular dystrophy in mdx mice.PLoS One. 2014 Sep 2;9(9):e106590. doi: 10.1371/journal.pone.0106590. eCollection 2014. PLoS One. 2014. PMID: 25181488 Free PMC article.
-
Dysregulation of Intracellular Ca2+ in Dystrophic Cortical and Hippocampal Neurons.Mol Neurobiol. 2018 Jan;55(1):603-618. doi: 10.1007/s12035-016-0311-7. Epub 2016 Dec 15. Mol Neurobiol. 2018. PMID: 27975174
-
Coenzyme Q10 supplementation acts as antioxidant on dystrophic muscle cells.Cell Stress Chaperones. 2019 Nov;24(6):1175-1185. doi: 10.1007/s12192-019-01039-2. Epub 2019 Oct 16. Cell Stress Chaperones. 2019. PMID: 31620981 Free PMC article.
-
Electrical stimuli are anti-apoptotic in skeletal muscle via extracellular ATP. Alteration of this signal in Mdx mice is a likely cause of dystrophy.PLoS One. 2013 Nov 25;8(11):e75340. doi: 10.1371/journal.pone.0075340. eCollection 2013. PLoS One. 2013. PMID: 24282497 Free PMC article.
References
-
- Blake DJ, Weir A, Newey SE, Davies KE (2002) Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev 82: 291–329. - PubMed
-
- Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, et al. (1987) Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 50: 509–517. - PubMed
-
- Emery AE (2002) The muscular dystrophies. Lancet 359: 687–695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

