Effect of alpha linolenic acid supplementation on serum prostate specific antigen (PSA): results from the alpha omega trial
- PMID: 24349086
- PMCID: PMC3859488
- DOI: 10.1371/journal.pone.0081519
Effect of alpha linolenic acid supplementation on serum prostate specific antigen (PSA): results from the alpha omega trial
Abstract
Background: Alpha linolenic acid (ALA) is the major omega-3 fatty acid in the diet. Evidence on health effects of ALA is not conclusive, but some observational studies found an increased risk of prostate cancer with higher intake of ALA. We examined the effect of ALA supplementation on serum concentrations of prostate-specific antigen (PSA), a biomarker for prostate cancer.
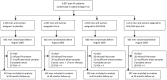
Methods: The Alpha Omega Trial (ClinicalTrials.gov Identifier: NCT00127452) was a double-blind, placebo-controlled trial of ALA and the fish fatty acids eicosapentanoic acid (EPA) and docosahexanoic acid (DHA) on the recurrence of cardiovascular disease, using a 2×2 factorial design. Blood was collected at the start and the end of the intervention period. The present analysis included 1622 patients with a history of a myocardial infarction, aged 60-80 years with an initial PSA concentration <4 ng/mL. They received either 2 g per day of ALA or placebo in margarine spreads for 40 months. T-tests and logistic regression were used to assess the effects of ALA supplementation on changes in serum PSA (both continuously and as a dichotomous outcome, cut-off point: >4 ng/mL).
Findings: Mean serum PSA increased by 0.42 ng/mL on placebo (n = 815) and by 0.52 ng/mL on ALA (n = 807), a difference of 0.10 (95% confidence interval: -0.02 to 0.22) ng/mL (P = 0·12). The odds ratio for PSA rising above 4 ng/mL on ALA versus placebo was 1.15 (95% CI: 0.84-1.58).
Interpretation: An additional amount of 2 g of ALA per day increased PSA by 0.10 ng/mL, but the confidence interval ranged from -0.02 to 0.22 ng/mL and included no effect. Therefore, more studies are needed to establish whether or not ALA intake has a clinically significant effect on PSA or prostate cancer.
Trial registration information: ClinicalTrials.gov; Identifier: NCT00127452. URL: http://www.clinicaltrials.gov/ct2/show/NCT00127452.
Conflict of interest statement
Figures


References
-
- Burdge GC, Jones AE, Wootton SA (2002) Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men. Br J Nutr 88: 355–363. - PubMed
-
- Goyens PL, Spilker ME, Zock PL, Katan MB, Mensink RP (2006) Conversion of alpha-linolenic acid in humans is influenced by the absolute amounts of alpha-linolenic acid and linoleic acid in the diet and not by their ratio. Am J Clin Nutr 84: 44–53. - PubMed
-
- Elmadfa I, Kornsteiner M (2009) Fats and fatty acid requirements for adults. Ann Nutr Metab 55: 56–75. - PubMed
-
- Simon JA, Chen YH, Bent S (2009) The relation of alpha-linolenic acid to the risk of prostate cancer: a systematic review and meta-analysis. Am J Clin Nutr 89: 1558S–1564S. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

