Swing-out of the β3 hybrid domain is required for αIIbβ3 priming and normal cytoskeletal reorganization, but not adhesion to immobilized fibrinogen
- PMID: 24349096
- PMCID: PMC3857192
- DOI: 10.1371/journal.pone.0081609
Swing-out of the β3 hybrid domain is required for αIIbβ3 priming and normal cytoskeletal reorganization, but not adhesion to immobilized fibrinogen
Abstract
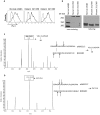
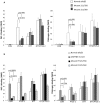
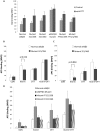
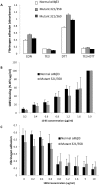
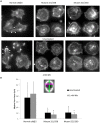
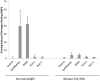
Structural and functional analyses of integrin αIIbβ3 has implicated swing-out motion of the β3 hybrid domain in αIIbβ3 activation and ligand binding. Using data from targeted molecular dynamics (TMD) simulations, we engineered two disulfide-bonded mutant receptors designed to limit swing-out (XS-O). XS-O mutants cannot bind the high Mr ligand fibrinogen in the presence of an activating mAb or after introducing mutations into the αIIb subunit designed to simulate inside-out signaling. They also have reduced capacity to be "primed" to bind fibrinogen by pretreatment with eptifibatide. They can, however, bind the small RGD venom protein kistrin. Despite their inability to bind soluble fibrinogen, the XS-O mutants can support adhesion to immobilized fibrinogen, although such adhesion does not initiate outside-in signaling leading to normal cytoskeletal reorganization. Collectively, our data further define the biologic role of β3 hybrid domain swing-out in both soluble and immobilized high Mr ligand binding, as well as in priming and outside-in signaling. We also infer that swing-out is likely to be a downstream effect of receptor extension.
Conflict of interest statement
Figures


Similar articles
-
An αIIbβ3 monoclonal antibody traps a semiextended conformation and allosterically inhibits large ligand binding.Blood Adv. 2024 Aug 27;8(16):4398-4409. doi: 10.1182/bloodadvances.2024013177. Blood Adv. 2024. PMID: 38968144 Free PMC article.
-
Effects of limiting extension at the alphaIIb genu on ligand binding to integrin alphaIIbbeta3.J Biol Chem. 2010 Jun 4;285(23):17604-13. doi: 10.1074/jbc.M110.107763. Epub 2010 Apr 2. J Biol Chem. 2010. PMID: 20363746 Free PMC article.
-
Regulation of integrin αIIbβ3 ligand binding and signaling by the metal ion binding sites in the β I domain.Biochemistry. 2011 Mar 29;50(12):2084-91. doi: 10.1021/bi2000092. Epub 2011 Feb 22. Biochemistry. 2011. PMID: 21309594
-
RGD, the Rho'd to cell spreading.Eur J Cell Biol. 2006 Apr;85(3-4):249-54. doi: 10.1016/j.ejcb.2005.08.003. Epub 2005 Sep 13. Eur J Cell Biol. 2006. PMID: 16546569 Review.
-
Beta3 tyrosine phosphorylation in alphaIIbbeta3 (platelet membrane GP IIb-IIIa) outside-in integrin signaling.Thromb Haemost. 2001 Jul;86(1):246-58. Thromb Haemost. 2001. PMID: 11487013 Review.
Cited by
-
αIIbβ3 binding to a fibrinogen fragment lacking the γ-chain dodecapeptide is activation dependent and EDTA inducible.Blood Adv. 2017 Feb 22;1(7):417-428. doi: 10.1182/bloodadvances.2017004689. eCollection 2017 Feb 28. Blood Adv. 2017. PMID: 29296957 Free PMC article.
-
Integrin Conformational Dynamics and Mechanotransduction.Cells. 2022 Nov 12;11(22):3584. doi: 10.3390/cells11223584. Cells. 2022. PMID: 36429013 Free PMC article. Review.
-
Molecular Basis of the Ligand Binding Specificity of αvβ8 Integrin.J Biol Chem. 2016 May 27;291(22):11551-65. doi: 10.1074/jbc.M116.719138. Epub 2016 Mar 31. J Biol Chem. 2016. PMID: 27033701 Free PMC article.
-
An αIIbβ3 monoclonal antibody traps a semiextended conformation and allosterically inhibits large ligand binding.Blood Adv. 2024 Aug 27;8(16):4398-4409. doi: 10.1182/bloodadvances.2024013177. Blood Adv. 2024. PMID: 38968144 Free PMC article.
-
Receptor-mediated cell mechanosensing.Mol Biol Cell. 2017 Nov 7;28(23):3134-3155. doi: 10.1091/mbc.E17-04-0228. Epub 2017 Sep 27. Mol Biol Cell. 2017. PMID: 28954860 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

