Carnosine's effect on amyloid fibril formation and induced cytotoxicity of lysozyme
- PMID: 24349167
- PMCID: PMC3859581
- DOI: 10.1371/journal.pone.0081982
Carnosine's effect on amyloid fibril formation and induced cytotoxicity of lysozyme
Abstract
Carnosine, a common dipeptide in mammals, has previously been shown to dissemble alpha-crystallin amyloid fibrils. To date, the dipeptide's anti-fibrillogensis effect has not been thoroughly characterized in other proteins. For a more complete understanding of carnosine's mechanism of action in amyloid fibril inhibition, we have investigated the effect of the dipeptide on lysozyme fibril formation and induced cytotoxicity in human neuroblastoma SH-SY5Y cells. Our study demonstrates a positive correlation between the concentration and inhibitory effect of carnosine against lysozyme fibril formation. Molecular docking results show carnosine's mechanism of fibrillogenesis inhibition may be initiated by binding with the aggregation-prone region of the protein. The dipeptide attenuates the amyloid fibril-induced cytotoxicity of human neuronal cells by reducing both apoptotic and necrotic cell deaths. Our study provides solid support for carnosine's amyloid fibril inhibitory property and its effect against fibril-induced cytotoxicity in SH-SY5Y cells. The additional insights gained herein may pave way to the discovery of other small molecules that may exert similar effects against amyloid fibril formation and its associated neurodegenerative diseases.
Conflict of interest statement
Figures
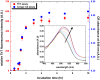
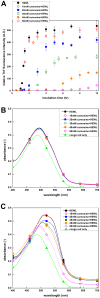

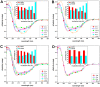
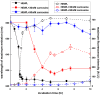




References
-
- Dobson CM (2004) Principles of protein folding, misfolding and aggregation. Semin Cell Dev Biol 15: 3–16. - PubMed
-
- Wang SSS, Good TA (2005) An overview of Alzheimer's disease. Journal of the Chinese Institute of Chemical Engineers 36: 533–559.
-
- Liberski PP, Sikorska B, Hauw JJ, Kopp N, Streichenberger N, et al. (2010) Ultrastructural Characteristics (or Evaluation) of Creutzfeldt-Jakob Disease and Other Human Transmissible Spongiform Encephalopathies or Prion Diseases. Ultrastructural Pathology 34: 351–361. - PubMed
-
- Ross CA, Poirier MA (2004) Protein aggregation and neurodegenerative disease. Nat Med 10 SupplS10–17. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

