Interleukin-encoding adenoviral vectors as genetic adjuvant for vaccination against retroviral infection
- PMID: 24349306
- PMCID: PMC3857891
- DOI: 10.1371/journal.pone.0082528
Interleukin-encoding adenoviral vectors as genetic adjuvant for vaccination against retroviral infection
Abstract
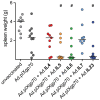
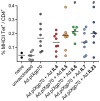
Interleukins (IL) are cytokines with stimulatory and modulatory functions in the immune system. In this study, we have chosen interleukins which are involved in the enhancement of TH2 responses and B cell functions to analyze their potential to improve a prophylactic adenovirus-based anti-retroviral vaccine with regard to antibody and virus-specific CD4(+) T cell responses. Mice were vaccinated with an adenoviral vector which encodes and displays the Friend Virus (FV) surface envelope protein gp70 (Ad.pIXgp70) in combination with adenoviral vectors encoding the interleukins IL4, IL5, IL6, IL7 or IL23. Co-application of Ad.pIXgp70 with Ad.IL5, Ad.IL6 or Ad.IL23 resulted in improved protection with high control over FV-induced splenomegaly and reduced viral loads. Mice co-immunized with adenoviral vectors encoding IL5 or IL23 showed increased neutralizing antibody responses while mice co-immunized with Ad.IL6 or Ad.IL23 showed improved FV-specific CD4(+) T cell responses compared to mice immunized with Ad.pIXgp70 alone. We show that the co-application of adenoviral vectors encoding specific interleukins is suitable to improve the vaccination efficacy of an anti-retroviral vaccine. Improved protection correlated with improved CD4(+) T cell responses and especially with higher neutralizing antibody titers. The co-application of selected interleukin-encoding adenoviral vectors is a valuable tool for vaccination with regard to enhancement of antibody mediated immunity.
Conflict of interest statement
Figures


Similar articles
-
Combination of nanoparticle-based therapeutic vaccination and transient ablation of regulatory T cells enhances anti-viral immunity during chronic retroviral infection.Retrovirology. 2016 Apr 14;13:24. doi: 10.1186/s12977-016-0258-9. Retrovirology. 2016. PMID: 27076190 Free PMC article. Review.
-
Vaccination with an adenoviral vector that encodes and displays a retroviral antigen induces improved neutralizing antibody and CD4+ T-cell responses and confers enhanced protection.J Virol. 2010 Feb;84(4):1967-76. doi: 10.1128/JVI.01840-09. Epub 2009 Dec 9. J Virol. 2010. PMID: 20007267 Free PMC article.
-
Improved vaccine protection against retrovirus infection after co-administration of adenoviral vectors encoding viral antigens and type I interferon subtypes.Retrovirology. 2011 Sep 26;8:75. doi: 10.1186/1742-4690-8-75. Retrovirology. 2011. PMID: 21943056 Free PMC article.
-
Comparative Evaluation of the Vaccine Efficacies of Three Adenovirus-Based Vector Types in the Friend Retrovirus Infection Model.J Virol. 2019 Oct 15;93(21):e01155-19. doi: 10.1128/JVI.01155-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31375593 Free PMC article.
-
Host genetic factors that control immune responses to retrovirus infections.Vaccine. 2008 Jun 6;26(24):2981-96. doi: 10.1016/j.vaccine.2008.01.004. Epub 2008 Jan 22. Vaccine. 2008. PMID: 18255203 Review.
Cited by
-
Induction of Potent and Long-Lived Antibody and Cellular Immune Responses in the Genitorectal Mucosa Could be the Critical Determinant of HIV Vaccine Efficacy.Front Immunol. 2014 May 8;5:202. doi: 10.3389/fimmu.2014.00202. eCollection 2014. Front Immunol. 2014. PMID: 24847327 Free PMC article. Review.
-
Genetic adjuvants: A paradigm shift in vaccine development and immune modulation.Mol Ther Nucleic Acids. 2025 Apr 8;36(2):102536. doi: 10.1016/j.omtn.2025.102536. eCollection 2025 Jun 10. Mol Ther Nucleic Acids. 2025. PMID: 40336572 Free PMC article. Review.
-
Induction of complex immune responses and strong protection against retrovirus challenge by adenovirus-based immunization depends on the order of vaccine delivery.Retrovirology. 2017 Feb 6;14(1):8. doi: 10.1186/s12977-017-0336-7. Retrovirology. 2017. PMID: 28166802 Free PMC article.
-
Filariae-Retrovirus Co-infection in Mice is Associated with Suppressed Virus-Specific IgG Immune Response and Higher Viral Loads.PLoS Negl Trop Dis. 2016 Dec 6;10(12):e0005170. doi: 10.1371/journal.pntd.0005170. eCollection 2016 Dec. PLoS Negl Trop Dis. 2016. PMID: 27923052 Free PMC article.
-
Combination of nanoparticle-based therapeutic vaccination and transient ablation of regulatory T cells enhances anti-viral immunity during chronic retroviral infection.Retrovirology. 2016 Apr 14;13:24. doi: 10.1186/s12977-016-0258-9. Retrovirology. 2016. PMID: 27076190 Free PMC article. Review.
References
-
- Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M et al. (2006) Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis 194: 1661-1671. doi:10.1086/508748. PubMed: 17109337. - DOI - PubMed
-
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R et al. (2008) Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372: 1881-1893. doi:10.1016/S0140-6736(08)61591-3. PubMed: 19012954. - DOI - PMC - PubMed
-
- National Institute of Allergy and Infectious Diseases (NIAID) (2013) NIH Discontinues Immunizations in HIV Vaccine Study. Available: http://www.hvtn.org/505-announcement-25April2013.html. Accessed 2013 November 6
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

