Human L-ficolin, a recognition molecule of the lectin activation pathway of complement, activates complement by binding to pneumolysin, the major toxin of Streptococcus pneumoniae
- PMID: 24349316
- PMCID: PMC3861440
- DOI: 10.1371/journal.pone.0082583
Human L-ficolin, a recognition molecule of the lectin activation pathway of complement, activates complement by binding to pneumolysin, the major toxin of Streptococcus pneumoniae
Abstract
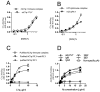
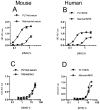
The complement system is an essential component of the immune response, providing a critical line of defense against different pathogens including S. pneumoniae. Complement is activated via three distinct pathways: the classical (CP), the alternative (AP) and the lectin pathway (LP). The role of Pneumolysin (PLY), a bacterial toxin released by S. pneumoniae, in triggering complement activation has been studied in vitro. Our results demonstrate that in both human and mouse sera complement was activated via the CP, initiated by direct binding of even non-specific IgM and IgG3 to PLY. Absence of CP activity in C1q(-/-) mouse serum completely abolished any C3 deposition. However, C1q depleted human serum strongly opsonized PLY through abundant deposition of C3 activation products, indicating that the LP may have a vital role in activating the human complement system on PLY. We identified that human L-ficolin is the critical LP recognition molecule that drives LP activation on PLY, while all of the murine LP recognition components fail to bind and activate complement on PLY. This work elucidates the detailed interactions between PLY and complement and shows for the first time a specific role of the LP in PLY-mediated complement activation in human serum.
Conflict of interest statement
Figures



References
-
- Miller E, Waight P, Efstratiou A, Brisson M, Johnson A, et al... (2000) Epidemiology of invasive and other pneumococcal disease in children in england and wales 1996–1998. Acta Paediatr Suppl 89: 11–16. - PubMed
-
- Yuste J, Botto M, Paton JC, Holden DW, Brown JS (2005) Additive inhibition of complement deposition by pneumolysin and PspA facilitates streptococcus pneumoniae septicemia. J Immunol 175: 1813–1819. - PubMed
-
- Kyaw MH, Christie P, Clarke SC, Mooney JD, Ahmed S, et al. (2003) Invasive pneumococcal disease in scotland, 1999–2001: Use of record linkage to explore associations between patients and disease in relation to future vaccination policy. Clin Infect Dis 37: 1283–1291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

