NFBD1/MDC1 is phosphorylated by PLK1 and controls G2/M transition through the regulation of a TOPOIIα-mediated decatenation checkpoint
- PMID: 24349352
- PMCID: PMC3859618
- DOI: 10.1371/journal.pone.0082744
NFBD1/MDC1 is phosphorylated by PLK1 and controls G2/M transition through the regulation of a TOPOIIα-mediated decatenation checkpoint
Abstract
Although it has been established that nuclear factor with BRCT domain 1/ mediator of the DNA damage checkpoint protein 1 (NFBD1/MDC1) is closely involved in DNA damage response, its possible contribution to the regulation of cell- cycle progression is unclear. In the present study, we have found for the first time that NFBD1 is phosphorylated by polo-like kinase 1 (PLK1) and has an important role in G2/M transition. Both NFBD1 and PLK1 are co-expressed in cellular nuclei throughout G2/M transition, and binding assays demonstrated direct interaction between NFBD1 and PLK1. Indeed, in vitro kinase reactions revealed that the PST domain of NFBD1 contains a potential amino acid sequence (845-DVTGEE-850) targeted by PLK1. Furthermore, enforced expression of GFP-PST but not GFP-PST(T847A) where threonine at 847 was substituted by alanine inhibited the phosphorylation levels of histone H3, suggesting a defect of M phase entry. Because PLK1 has been implicated in promoting the G2/M transition, we reasoned that overexpressed PST might serve as a pseudosubstrate for PLK1 and thus interfere with phosphorylation of endogenous PLK1 substrates. Interestingly, siRNA-mediated knockdown of NFBD1 resulted in early M phase entry and accelerated M phase progression, raising the possibility that NFBD1 is a PLK1 substrate for regulating the G2/M transition. Moreover, the constitutive active form of PLK1(T210D) overcame the ICRF-193-induced decatenation checkpoint and inhibited the interaction between NFBD1 and topoisomerase IIα, but kinase-deficient PLK1 did not. Based on these observations, we propose that PLK1-mediated phosphorylation of NFBD1 is involved in the regulation of G2/M transition by recovering a decatenation checkpoint.
Conflict of interest statement
Figures

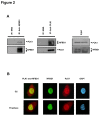


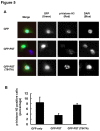
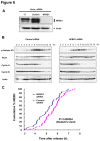
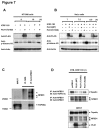
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

