S-1-based chemotherapy versus capecitabine-based chemotherapy as first-line treatment for advanced gastric carcinoma: a meta-analysis
- PMID: 24349363
- PMCID: PMC3861463
- DOI: 10.1371/journal.pone.0082798
S-1-based chemotherapy versus capecitabine-based chemotherapy as first-line treatment for advanced gastric carcinoma: a meta-analysis
Abstract
Background: Although both oral fluoropyrimidines were reported effective and safe, doubts exist about whether S-1 or capecitabine is more advantageous in advanced gastric carcinoma (AGC). Herein, we performed a meta-analysis to comprehensively compare the efficacy and safety of S-1-based chemotherapy versus capecitabine-based chemotherapy as first-line treatment for AGC.
Methods: PubMed/Medline, EmBase, Cochrane library, and China National Knowledge Infrastructure databases were searched for articles comparing S-1-based chemotherapy to capecitabine-based chemotherapy for AGC. Primary outcomes were overall response rate (ORR), time to progression (TTP), overall survival (OS), progression-free probability, and survival probability. Secondary outcomes were toxicities. Fixed-effects model were used and all the results were confirmed by random-effects model.
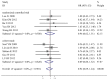
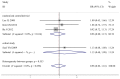
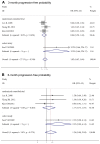
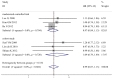
Results: Five randomized controlled trials and five cohort studies with 821 patients were included. We found equivalent ORR (38.3% vs. 39.1%, odds ratio [OR] 0.92, 95% confidence interval [CI] 0.69-1.24, P = 0.59), TTP (harzad ratio [HR] 0.98, 95% CI 0.82-1.16, P = 0.79), OS (HR 0.99, 95% CI 0.87-1.13, P = 0.91), progression-free probability (3-month OR 1.02, 95% CI 0.62-1.68, P = 0.94; 6-month OR 1.34, 95% CI 0.88-2.04, P = 0.18) and survival probability (0.5-year OR 0.90, 95% CI 0.61-1.31, P =0.57; 1-year OR 0.97, 95% CI 0.70- 1.33, P = 0.84; 2-year OR 1.15, 95% CI 0.61-2.17, P = 0.66). Equivalent grade 3 to 4 hematological and non-hematological toxicities were found except hand-foot syndrome was less prominent in S-1-based chemotherapy (0.3% vs. 5.9%, OR 0.19, 95% CI 0.06-0.56, P = 0.003). There're no significant heterogeneity and publication bias. Cumulative analysis found stable time-dependent trend. Consistent results stratified by study design, age, regimen, cycle, country were observed.
Conclusion: S-1-based chemotherapy was associated with non-inferior antitumor efficacy and better safety profile, compared with capecitabine-based therapy. We recommended S-1 and capecitabine can be used interchangeably for AGC, at least in Asia.
Conflict of interest statement
Figures




References
-
- Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24: 2137-2150. doi:10.1200/JCO.2005.05.2308. PubMed: 16682732. - DOI - PubMed
-
- Chen XL, Chen XZ, Yang C, Liao YB, Li H, et al. (2013) Docetaxel, Cisplatin and Fluorouracil (DCF) Regimen Compared with Non-Taxane-Containing Palliative Chemotherapy for Gastric Carcinoma: A Systematic Review and Meta-Analysis. PLoS One 8: e60320. doi:10.1371/journal.pone.0060320. - DOI - PMC - PubMed
-
- Van Cutsem E, Van de Velde C, Roth A, Lordick F, Köhne C-H et al. (2008) Expert opinion on management of gastric and gastro-oesophageal junction adenocarcinoma on behalf of the European Organisation for Research and Treatment of Cancer (EORTC)-gastrointestinal cancer group. Eur J Cancer 44: 182-194. doi:10.1016/j.ejca.2007.11.001. PubMed: 18093827. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

