Effects of vitamin A on in vitro maturation of pre-pubertal mouse spermatogonial stem cells
- PMID: 24349372
- PMCID: PMC3857286
- DOI: 10.1371/journal.pone.0082819
Effects of vitamin A on in vitro maturation of pre-pubertal mouse spermatogonial stem cells
Abstract
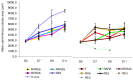
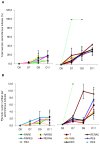
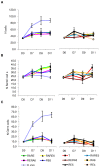
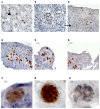
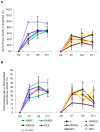
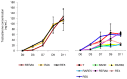
Testicular tissue cryopreservation is the only potential option for fertility preservation in pre-pubertal boys exposed to gonadotoxic treatment. Completion of spermatogenesis after in vitro maturation is one of the future uses of harvested testicular tissue. The purpose of the current study was to evaluate the effects of vitamin A on in vitro maturation of fresh and frozen-thawed mouse pre-pubertal spermatogonial stem cells in an organ culture system. Pre-pubertal CD1 mouse fresh testes were cultured for 7 (D7), 9 (D9) and 11 (D11) days using an organ culture system. Basal medium was supplemented with different concentrations of retinol (Re) or retinoic acid (RA) alone or in combination. Seminiferous tubule morphology (tubule diameter, intra-tubular cell type), intra-tubular cell death and proliferation (PCNA antibody) and testosterone level were assessed at D7, D9 and D11. Pre-pubertal mouse testicular tissue were frozen after a soaking temperature performed at -7 °C, -8 °C or -9 °C and after thawing, were cultured for 9 days, using the culture medium preserving the best fresh tissue functionality. Retinoic acid at 10(-6)M and retinol at 3.3.10(-7)M, as well as retinol 10(-6)M are favourable for seminiferous tubule growth, maintenance of intra-tubular cell proliferation and germ cell differentiation of fresh pre-pubertal mouse spermatogonia. Structural and functional integrity of frozen-thawed testicular tissue appeared to be well-preserved after soaking temperature at -8 °C, after 9 days of organotypic culture using 10(-6)M retinol. RA and Re can control in vitro germ cell proliferation and differentiation. Re at a concentration of 10(-6)M maintains intra-tubular cell proliferation and the ability of spermatogonia to initiate spermatogenesis in fresh and frozen pre-pubertal mouse testicular tissue using a soaking temperature at -8 °C. Our data suggested a possible human application for in vitro maturation of cryopreserved pre-pubertal testicular tissue.
Conflict of interest statement
Figures




References
-
- Menon S, Rives N, Mousset-Siméon N, Sibert L, Vannier JP et al. (2009) Fertility preservation in adolescent males: experience over 22 years at Rouen University Hospital. Hum. Reprod 24: 37-44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

