Is sustained virological response a marker of treatment efficacy in patients with chronic hepatitis C viral infection with no response or relapse to previous antiviral intervention?
- PMID: 24349487
- PMCID: PMC3861485
- DOI: 10.1371/journal.pone.0083313
Is sustained virological response a marker of treatment efficacy in patients with chronic hepatitis C viral infection with no response or relapse to previous antiviral intervention?
Abstract
Background: Randomised clinical trials (RCTs) of antiviral interventions in patients with chronic hepatitis C virus (HCV) infection use sustained virological response (SVR) as the main outcome. There is sparse information on long-term mortality from RCTs.
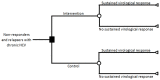
Methods: We created a decision tree model based on a Cochrane systematic review on interferon retreatment for patients who did not respond to initial therapy or who relapsed following SVR. Extrapolating data to 20 years, we modelled the outcome from three scenarios: (1) observed medium-term (5 year) annual mortality rates continue to the long term (20 years); (2) long-term annual mortality in retreatment responders falls to that of the general population while retreatment non-responders continue at the medium-term mortality; (3) long-term annual mortality in retreatment non-responders is the same as control group non-responders (i.e., the increased treatment-related medium mortality "wears off").
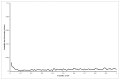
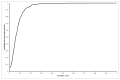
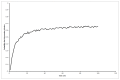
Results: The mean differences in life expectancy over 20 years with interferon versus control in the first, second, and third scenarios were -0.34 years (95% confidence interval (CI) -0.71 to 0.03), -0.23 years (95% CI -0.69 to 0.24), and -0.01 (95% CI -0.3 to 0.27), respectively. The life expectancy was always lower in the interferon group than in the control group in scenario 1. In scenario 3, the interferon group had a longer life expectancy than the control group only when more than 7% in the interferon group achieved SVR.
Conclusions: SVR may be a good prognostic marker but does not seem to be a valid surrogate marker for assessing HCV treatment efficacy of interferon retreatment. The SVR threshold at which retreatment increases life expectancy may be different for different drugs depending upon the adverse event profile and treatment efficacy. This has to be determined for each drug by RCTs and appropriate modelling before SVR can be accepted as a surrogate marker.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

