Mannitol enhances antibiotic sensitivity of persister bacteria in Pseudomonas aeruginosa biofilms
- PMID: 24349568
- PMCID: PMC3862834
- DOI: 10.1371/journal.pone.0084220
Mannitol enhances antibiotic sensitivity of persister bacteria in Pseudomonas aeruginosa biofilms
Abstract
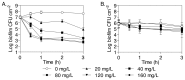
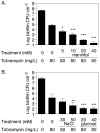
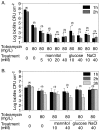
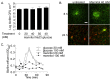
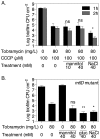
The failure of antibiotic therapies to clear Pseudomonas aeruginosa lung infection, the key mortality factor for cystic fibrosis (CF) patients, is partly attributed to the high tolerance of P. aeruginosa biofilms. Mannitol has previously been found to restore aminoglycoside sensitivity in Escherichia coli by generating a proton-motive force (PMF), suggesting a potential new strategy to improve antibiotic therapy and reduce disease progression in CF. Here, we used the commonly prescribed aminoglycoside tobramycin to select for P. aeruginosa persister cells during biofilm growth. Incubation with mannitol (10-40 mM) increased tobramycin sensitivity of persister cells up to 1,000-fold. Addition of mannitol to pre-grown biofilms was able to revert the persister phenotype and improve the efficacy of tobramycin. This effect was blocked by the addition of a PMF inhibitor or in a P. aeruginosa mutant strain unable to metabolise mannitol. Addition of glucose and NaCl at high osmolarity also improved the efficacy of tobramycin although to a lesser extent compared to mannitol. Therefore, the primary effect of mannitol in reverting biofilm associated persister cells appears to be an active, physiological response, associated with a minor contribution of osmotic stress. Mannitol was tested against clinically relevant strains, showing that biofilms containing a subpopulation of persister cells are better killed in the presence of mannitol, but a clinical strain with a high resistance to tobramycin was not affected by mannitol. Overall, these results suggest that in addition to improvements in lung function by facilitating mucus clearance in CF, mannitol also affects antibiotic sensitivity in biofilms and does so through an active, physiological response.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

