Development and evaluation of 6-mercaptopurine and metoclopramide polypill formulation for oral administration: In-vitro and ex vivo studies
- PMID: 24350042
- PMCID: PMC3853762
- DOI: 10.4103/2230-973X.121306
Development and evaluation of 6-mercaptopurine and metoclopramide polypill formulation for oral administration: In-vitro and ex vivo studies
Abstract
Introduction: The present investigation was to develop a polypill of 6-mercaptopurine and metoclopramide. A polypill with delayed release granules of an anticancer and immediate release mucoadhesive tablet of antiemetic may result in the reduction of emesis caused by oral chemotherapy.
Materials and methods: 6-Mercaptopurine granules were prepared by wet granulation process. Chitosan, hydroxypropyl methylcellulose, and ethylcellulose were used as individually as delayed release polymers. Seven granule formulations (F1-F7) were prepared and evaluated for flow properties and drug content. Immediate release mucoadhesive tablets of metoclopramide were prepared by direct compression technique using pectin and PVPK-40 as mucoadhesive polymers. Three formulations of pectin (L1-L3) and three formulations of PVPK40 (M1-M3) were prepared using lactose, magnesium stearate, and mannitol and talc as diluent and glidant, respectively. Tablets were evaluated for weight variation, hardness, friability, drug content, ex vivo mucoadhesion time, and in vitro dissolution studies.
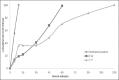
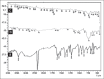
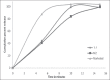
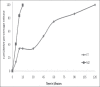
Results: Formulation F2, F4, F5, and F7 showed maximum drug content. Formulation F7 exhibited the drug release up to 2 h and was selected as the best delayed release formulation. All formulations of metoclopramide showed good drug content ranging from 97.6 % to 100.6%. Formulation M2 among tablets prepared with PVP exhibited desired mucoadhesion time of 15.33 min which prolongs the duration of drug release in gastric pouch of the male Wistar rats. Both the selected formulations F7 and M2 were filled into body of capsule size 0 and capsule was evaluated for technological properties.
Conclusion: It may be concluded that polypill released the metoclopramide immediately prior to 6-mercaptopurine.
Keywords: 6-mercaptopurine; delayed release; immediate release; metoclopramide; polypill.
Conflict of interest statement
Figures
Similar articles
-
Formulation, development, and performance evaluation of metoclopramide HCl oro-dispersible sustained release tablet.Arch Pharm Res. 2011 Oct;34(10):1691-700. doi: 10.1007/s12272-011-1013-3. Epub 2011 Nov 12. Arch Pharm Res. 2011. PMID: 22076769
-
Effect of Different Polymer Concentration on Drug Release Rate and Physicochemical Properties of Mucoadhesive Gastroretentive Tablets.Indian J Pharm Sci. 2015 Nov-Dec;77(6):705-14. doi: 10.4103/0250-474x.174993. Indian J Pharm Sci. 2015. PMID: 26997698 Free PMC article.
-
Development and evaluation of hydrophilic colloid matrix of famotidine tablets.AAPS PharmSciTech. 2010 Jun;11(2):708-18. doi: 10.1208/s12249-010-9427-7. Epub 2010 Apr 27. AAPS PharmSciTech. 2010. PMID: 20422332 Free PMC article.
-
In vitro and in vivo evaluation of hydrophilic and hydrophobic polymers-based nicorandil-loaded peroral tablet compared with its once-daily commercial sustained-release tablet.Drug Dev Ind Pharm. 2011 Apr;37(4):436-45. doi: 10.3109/03639045.2010.521161. Epub 2010 Oct 6. Drug Dev Ind Pharm. 2011. PMID: 20923389
-
Propranolol Hydrochloride Buccoadhesive Tablet: Development and In-vitro Evaluation.Iran J Pharm Res. 2020 Spring;19(2):22-33. doi: 10.22037/ijpr.2019.13866.13346. Iran J Pharm Res. 2020. PMID: 33224208 Free PMC article.
References
-
- Navarro RP, Morrow T, Baran R. Pharmacoeconomic and clinical outcomes in oncology using oral chemotherapy. Manag Care Interface. 2002;15:55–62. - PubMed
-
- Mi FL, Chen CT, Tseng YC, Kuan CY, Shyu SS. Iron (3) carboxymethyl chitin microsphere for the Ph- sensitive release of 6-Mercaptopurine. J Control Rel. 1997;44:19–32.
-
- Schnell FM. Chemotherapy-induced nausea and vomiting: The importance of acute antiemetic control. Oncologist. 2003;8:187–98. - PubMed
-
- Olver IN. Updates on antiemetics for chemotherapy-induced emesis. Intern Med J. 2005;35:478–81. - PubMed
-
- Gurunberg SM. Cost-effective use of antiemetics. Oncology. 1998;12:38–42. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

