Evaluation of in vivo efficacy and toxicity of prednisolone-loaded hydrogel-based drug delivery device
- PMID: 24350043
- PMCID: PMC3853763
- DOI: 10.4103/2230-973X.121308
Evaluation of in vivo efficacy and toxicity of prednisolone-loaded hydrogel-based drug delivery device
Abstract
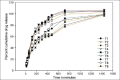
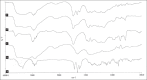
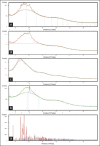
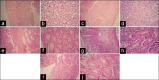
Drugs prescribed for the treatment of moderate to severe inflammatory bowel disease (IBD) are associated with number of side effects. Targeted drug delivery is essential for the treatment of inflammatory bowel disease in order to increase efficacy and reduce toxicity. The established delivery system is designed on enzyme and time-based release of poorly soluble prednisolone, a drug of choice for the treatment of moderate to severe inflammatory bowel disease. Their pharmacological evaluation was done in 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced model of colitis in rat. The drug was administered once daily for 3 consecutive days. Visible severity of colitis, tissue to bodyweight ratio, tissue histology along with nitric oxide (NO), malondialdehyde (MDA) and myeloperoxidase (MPO) activity of colonic tissue were studied to estimate the efficacy of the drug-loaded delivery system. The highest efficacy was observed for formulation in which Eudragit RS100 (EU) was used along with guar gum (GG) in a ratio 2:5 for the preparation of delivery device. An effective recovery was observed from the study of tissue histology of animals treated with the drug-loaded optimized formulation and the biochemical parameters supported it. The toxicity of prednisolone (PD) was reduced significantly as predicted from thymus to body weight ratio of treated animals. GG and EU RS100 provided a newer bipolymer combination for the colon-targeted delivery of PD which increased its efficacy and reduced the toxic side effects. The in vivo experiments presented effective amelioration from colitis in TNBS-induced animal model of colitis.
Keywords: Colon; TNBS colitis; eudragit RS100; guar gum; prednisolone.
Conflict of interest statement
Figures



Similar articles
-
Efficacy and toxicity of Eudragit-coated chitosan-succinyl-prednisolone conjugate microspheres using rats with 2,4,6-trinitrobenzenesulfonic acid-induced colitis.Int J Pharm. 2008 Jun 24;358(1-2):296-302. doi: 10.1016/j.ijpharm.2008.02.015. Epub 2008 Mar 10. Int J Pharm. 2008. PMID: 18394832
-
Partially hydrolysed guar gum ameliorates murine intestinal inflammation in association with modulating luminal microbiota and SCFA.Br J Nutr. 2016 Oct;116(7):1199-1205. doi: 10.1017/S0007114516003068. Epub 2016 Sep 8. Br J Nutr. 2016. PMID: 27604176
-
Application of an amine functionalized biopolymer in the colonic delivery of glycyrrhizin: a design and in vivo efficacy study.Sci Pharm. 2013 May 18;81(4):1101-22. doi: 10.3797/scipharm.1301-14. Print 2013 Oct-Dec. Sci Pharm. 2013. PMID: 24482776 Free PMC article.
-
Preclinical Study in Vivo for New Pharmacological Approaches in Inflammatory Bowel Disease: A Systematic Review of Chronic Model of TNBS-Induced Colitis.J Clin Med. 2019 Oct 1;8(10):1574. doi: 10.3390/jcm8101574. J Clin Med. 2019. PMID: 31581545 Free PMC article. Review.
-
[Study on the colon specific delivery of prednisolone using chitosan capsules].Yakugaku Zasshi. 2007 Apr;127(4):621-30. doi: 10.1248/yakushi.127.621. Yakugaku Zasshi. 2007. PMID: 17409691 Review. Japanese.
Cited by
-
In Vitro and In Vivo Correlation of Colon-Targeted Compression-Coated Tablets.J Pharm (Cairo). 2016;2016:5742967. doi: 10.1155/2016/5742967. Epub 2016 Feb 17. J Pharm (Cairo). 2016. PMID: 26989562 Free PMC article.
References
-
- Ray G. Inflammatory bowel disease in India--changing paradigms. Int J Colorectal Dis. 2011;26:635–44. - PubMed
-
- Probert CS, Mayberry JF, Mann R. Inflammatory bowel disease in the rural Indian subcontinent: A survey of patients attending mission hospitals. Digestion. 1990;47:42–6. - PubMed
-
- Thia KT, Loftus EV, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008;103:3167–82. - PubMed
-
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

