Beyond DNA Repair: Additional Functions of PARP-1 in Cancer
- PMID: 24350055
- PMCID: PMC3841914
- DOI: 10.3389/fonc.2013.00290
Beyond DNA Repair: Additional Functions of PARP-1 in Cancer
Abstract
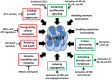
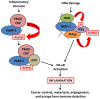
Poly(ADP-ribose) polymerases (PARPs) are DNA-dependent nuclear enzymes that transfer negatively charged ADP-ribose moieties from cellular nicotinamide-adenine-dinucleotide (NAD(+)) to a variety of protein substrates, altering protein-protein and protein-DNA interactions. The most studied of these enzymes is poly(ADP-ribose) polymerase-1 (PARP-1), which is an excellent therapeutic target in cancer due to its pivotal role in the DNA damage response. Clinical studies have shown susceptibility to PARP inhibitors in DNA repair defective cancers with only mild adverse side effects. Interestingly, additional studies are emerging which demonstrate a role for this therapy in DNA repair proficient tumors through a variety of mechanisms. In this review, we will discuss additional functions of PARP-1 - including regulation of inflammatory mediators, cellular energetics and death pathways, gene transcription, sex hormone- and ERK-mediated signaling, and mitosis - and the role these PARP-1-mediated processes play in oncogenesis, cancer progression, and the development of therapeutic resistance. As PARP-1 can act in both a pro- and anti-tumor manner depending on the context, it is important to consider the global effects of this protein in determining when, and how, to best use PARP inhibitors in anticancer therapy.
Keywords: ERK signaling; NF-κB; PARP inhibitors; PARP-1; angiogenesis; genetic transcription; mitotic spindle; sex hormone signaling.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

