Role of reactive oxygen species in neonatal pulmonary vascular disease
- PMID: 24350610
- PMCID: PMC4202910
- DOI: 10.1089/ars.2013.5785
Role of reactive oxygen species in neonatal pulmonary vascular disease
Abstract
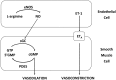
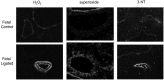
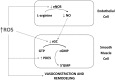
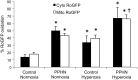
Significance: Abnormal lung development in the perinatal period can result in severe neonatal complications, including persistent pulmonary hypertension (PH) of the newborn and bronchopulmonary dysplasia. Reactive oxygen species (ROS) play a substantive role in the development of PH associated with these diseases. ROS impair the normal pulmonary artery (PA) relaxation in response to vasodilators, and ROS are also implicated in pulmonary arterial remodeling, both of which can increase the severity of PH.
Recent advances: PA ROS levels are elevated when endogenous ROS-generating enzymes are activated and/or when endogenous ROS scavengers are inactivated. Animal models have provided valuable insights into ROS generators and scavengers that are dysregulated in different forms of neonatal PH, thus identifying potential therapeutic targets.
Critical issues: General antioxidant therapy has proved ineffective in reversing PH, suggesting that it is necessary to target specific signaling pathways for successful therapy.
Future directions: Development of novel selective pharmacologic inhibitors along with nonantioxidant therapies may improve the treatment outcomes of patients with PH, while further investigation of the underlying mechanisms may enable earlier detection of the disease.
Figures



References
-
- Abman SH. Impaired vascular endothelial growth factor signaling in the pathogenesis of neonatal pulmonary vascular disease. Adv Exp Med Biol 661: 323–335, 2010 - PubMed
-
- Afolayan AJ, Eis A, Teng RJ, Bakhutashvili I, Kaul S, Davis JM, and Konduri GG. Decreases in manganese superoxide dismutase expression and activity contribute to oxidative stress in persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 303: L870–L879, 2012 - PMC - PubMed
-
- Afshar S, Gibson LL, Yuhanna IS, Sherman TS, Kerecman JD, Grubb PH, Yoder BA, McCurnin DC, and Shaul PW. Pulmonary NO synthase expression is attenuated in a fetal baboon model of chronic lung disease. Am J Physiol Lung Cell Mol Physiol 284: L749–L758, 2003 - PubMed
-
- Ahmed MN, Suliman HB, Folz RJ, Nozik-Grayck E, Golson ML, Mason SN, and Auten RL. Extracellular superoxide dismutase protects lung development in hyperoxia-exposed newborn mice. Am J Respir Crit Care Med 167: 400–405, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

