Chemical pyrophosphorylation of functionally diverse peptides
- PMID: 24350643
- PMCID: PMC3992712
- DOI: 10.1021/ja411737c
Chemical pyrophosphorylation of functionally diverse peptides
Abstract
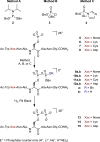
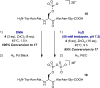
A highly selective and convenient method for the synthesis of pyrophosphopeptides in solution is reported. The remarkable compatibility with functional groups (alcohol, thiol, amine, carboxylic acid) in the peptide substrates suggests that the intrinsic nucleophilicity of the phosphoserine residue is much higher than previously appreciated. Because the methodology operates in polar solvents, including water, a broad range of pyrophosphopeptides can be accessed. We envision these peptides will find widespread applications in the development of mass spectrometry and antibody-based detection methods for pyrophosphoproteins.
Figures

References
-
- Walsh CT. Posttranslational Modifications of Proteins: Expanding Nature's Inventory. 1. Roberts and Co. Publishers; 2005.
-
-
For recent reviews on diphosphoinositol polyphosphate signaling see:
- Wilson MSC, Livermore TM, Saiardi A. Biochem J. 2013;452:369. - PubMed
- Wundenberg T, Mayr GW. Biol Chem. 2012;393:979. - PubMed
- Chakraborty A, Kim S, Snyder SH. Sci Signal. 2011;4 - PMC - PubMed
- Shears SB, Gokhale NA, Wang H, Zaremba A. Adv Enzyme Regul. 2011;51:13. - PMC - PubMed
-
-
- Saiardi A, Bhandari R, Resnick AC, Snowman AM, Snyder SH. Science. 2004;306:2101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

