Genotype-dependency of butyrate efficacy in children with congenital chloride diarrhea
- PMID: 24350656
- PMCID: PMC3878237
- DOI: 10.1186/1750-1172-8-194
Genotype-dependency of butyrate efficacy in children with congenital chloride diarrhea
Abstract
Background: Congenital chloride diarrhea (CLD) is an autosomal recessive disorder characterized by life-long, severe diarrhea with intestinal Cl- malabsorption. It results from a reduced activity of the down regulated in adenoma exchanger (DRA), due to mutations in the solute carrier family 26, member 3 (SLC26A3) gene. Currently available therapies are not able to limit the severity of diarrhea in CLD. Conflicting results have been reported on the therapeutic efficacy of oral butyrate.
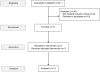
Methods: We investigated the effect of oral butyrate (100 mg/kg/day) in seven CLD children with different SLC26A3 genotypes. Nasal epithelial cells were obtained to assess the effect of butyrate on the expression of the two main Cl- transporters: DRA and putative anion transporter-1 (PAT-1).
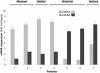
Results: A variable clinical response to butyrate was observed regarding the stool pattern and fecal ion loss. The best response was observed in subjects with missense and deletion mutations. Variable response to butyrate was also observed on SLC26A3 (DRA) and SLC26A6 (PAT1) gene expression in nasal epithelial cells of CLD patients.
Conclusions: We demonstrate a genotype-dependency for butyrate therapeutic efficacy in CLD. The effect of butyrate is related in part on a different modulation of the expression of the two main apical membrane Cl- exchangers of epithelial cells, members of the SLC26 anion family.
Trial registration: Australian New Zealand Clinical trial Registry ACTRN12613000450718.
Figures
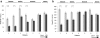
References
-
- Berni Canani R, Terrin G, Cardillo G, Tomaiuolo R, Castaldo G. Congenital diarrheal disorders: improved understanding of gene defects is leading to advances in intestinal physiology and clinical management. J Pediatr Gastroenterol Nutr. 2010;8:360–366. - PubMed
-
- Berni Canani R, Terrin G. Recent progress in congenital diarrheal disorders. Curr Gastroenterol Rep. 2011;8:257–264. - PubMed
-
- Hoglund P, Auranen M, Socha J, Popinska K, Nazer H, Rajaram U, Al Sanie A, Al-Ghanim M, Holmberg C, de la Chapelle A, Kere J. Genetic background of congenital chloride diarrhea in high incidence populations: Finland, Poland, and Saudi Arabia and Kuwait. Am J Hum Genet. 1998;8:760–768. - PMC - PubMed
-
- Lechner S, Ruemmele FM, Zankl A, Lausch E, Huber WD, Mihatsch W, Phillips AD, Lewindon P, Querfeld U, Heinz-Erian P, Müller T, Janecke AR. Significance of molecular testing for congenital chloride diarrhea. J Pediatr Gastroenterol Nutr. 2011;8:48–54. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

