A central role for dityrosine crosslinking of Amyloid-β in Alzheimer's disease
- PMID: 24351276
- PMCID: PMC3880074
- DOI: 10.1186/2051-5960-1-83
A central role for dityrosine crosslinking of Amyloid-β in Alzheimer's disease
Abstract
Background: Alzheimer's disease (AD) is characterized by the deposition of insoluble amyloid plaques in the neuropil composed of highly stable, self-assembled Amyloid-beta (Aβ) fibrils. Copper has been implicated to play a role in Alzheimer's disease. Dimers of Aβ have been isolated from AD brain and have been shown to be neurotoxic.
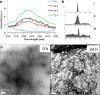
Results: We have investigated the formation of dityrosine cross-links in Aβ42 formed by covalent ortho-ortho coupling of two tyrosine residues under conditions of oxidative stress with elevated copper and shown that dityrosine can be formed in vitro in Aβ oligomers and fibrils and that these links further stabilize the fibrils. Dityrosine crosslinking was present in internalized Aβ in cell cultures treated with oligomeric Aβ42 using a specific antibody for dityrosine by immunogold labeling transmission electron microscopy. Results also revealed the prevalence of dityrosine crosslinks in amyloid plaques in brain tissue and in cerebrospinal fluid from AD patients.
Conclusions: Aβ dimers may be stabilized by dityrosine crosslinking. These results indicate that dityrosine cross-links may play an important role in the pathogenesis of Alzheimer's disease and can be generated by reactive oxygen species catalyzed by Cu2+ ions. The observation of increased Aβ and dityrosine in CSF from AD patients suggests that this could be used as a potential biomarker of oxidative stress in AD.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

