Outcomes and complications of intracranial pressure monitoring in acute liver failure: a retrospective cohort study
- PMID: 24351370
- PMCID: PMC4563984
- DOI: 10.1097/CCM.0000000000000144
Outcomes and complications of intracranial pressure monitoring in acute liver failure: a retrospective cohort study
Abstract
Objective: To determine if intracranial pressure monitor placement in patients with acute liver failure is associated with significant clinical outcomes.
Design: Retrospective multicenter cohort study.
Setting: Academic liver transplant centers comprising the U.S. Acute Liver Failure Study Group.
Patients: Adult critically ill patients with acute liver failure presenting with grade III/IV hepatic encephalopathy (n = 629) prospectively enrolled between March 2004 and August 2011.
Intervention: Intracranial pressure monitored (n = 140) versus nonmonitored controls (n = 489).
Measurements and main results: Intracranial pressure monitored patients were younger than controls (35 vs 43 yr, p < 0.001) and more likely to be on renal replacement therapy (52% vs 38%, p = 0.003). Of 87 intracranial pressure monitored patients with detailed information, 44 (51%) had evidence of intracranial hypertension (intracranial pressure > 25 mm Hg) and overall 21-day mortality was higher in patients with intracranial hypertension (43% vs 23%, p = 0.05). During the first 7 days, intracranial pressure monitored patients received more intracranial hypertension-directed therapies (mannitol, 56% vs 21%; hypertonic saline, 14% vs 7%; hypothermia, 24% vs 10%; p < 0.03 for each). Forty-one percent of intracranial pressure monitored patients received liver transplant (vs 18% controls; p < 0.001). Overall 21-day mortality was similar (intracranial pressure monitored 33% vs controls 38%, p = 0.24). Where data were available, hemorrhagic complications were rare in intracranial pressure monitored patients (4 of 56 [7%]; three died). When stratifying by acetaminophen status and adjusting for confounders, intracranial pressure monitor placement did not impact 21-day mortality in acetaminophen patients (p = 0.89). However, intracranial pressure monitor was associated with increased 21-day mortality in nonacetaminophen patients (odds ratio, ~ 3.04; p = 0.014).
Conclusions: In intracranial pressure monitored patients with acute liver failure, intracranial hypertension is commonly observed. The use of intracranial pressure monitor in acetaminophen acute liver failure did not confer a significant 21-day mortality benefit, whereas in nonacetaminophen acute liver failure, it may be associated with worse outcomes. Hemorrhagic complications from intracranial pressure monitor placement were uncommon and cannot account for mortality trends. Although our results cannot conclusively confirm or refute the utility of intracranial pressure monitoring in patients with acute liver failure, patient selection and ancillary assessments of cerebral blood flow likely have a significant role. Prospective studies would be required to conclusively account for confounding by illness severity and transplant.
Conflict of interest statement
Figures
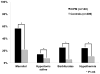
Types of ICP therapies received during first 7 days
Therapies: mannitol, hypertonic saline, barbiturates, hypothermia
Missing data listed in Table 1
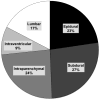
Where data available after querying of US ALFSG sites
Data reported as percentages
Comment in
-
Intracranial pressure in acute liver failure: to bolt or not to bolt-that is the question.Crit Care Med. 2014 May;42(5):1304-5. doi: 10.1097/CCM.0000000000000242. Crit Care Med. 2014. PMID: 24736348 No abstract available.
Similar articles
-
Invasive intracranial pressure monitoring is a useful adjunct in the management of severe hepatic encephalopathy associated with pediatric acute liver failure.Pediatr Crit Care Med. 2012 Jan;13(1):e33-8. doi: 10.1097/PCC.0b013e31820ac08f. Pediatr Crit Care Med. 2012. PMID: 21263362 Free PMC article.
-
Complications and use of intracranial pressure monitoring in patients with acute liver failure and severe encephalopathy.Liver Transpl. 2005 Dec;11(12):1581-9. doi: 10.1002/lt.20625. Liver Transpl. 2005. PMID: 16315300
-
Intracranial pressure in acute liver failure: to bolt or not to bolt-that is the question.Crit Care Med. 2014 May;42(5):1304-5. doi: 10.1097/CCM.0000000000000242. Crit Care Med. 2014. PMID: 24736348 No abstract available.
-
Assessment and management of cerebral edema and intracranial hypertension in acute liver failure.J Crit Care. 2013 Oct;28(5):783-91. doi: 10.1016/j.jcrc.2013.04.002. Epub 2013 May 15. J Crit Care. 2013. PMID: 23683564 Review.
-
Brain edema and intracranial hypertension in fulminant hepatic failure: pathophysiology and management.World J Gastroenterol. 2006 Dec 14;12(46):7405-12. doi: 10.3748/wjg.v12.i46.7405. World J Gastroenterol. 2006. PMID: 17167826 Free PMC article. Review.
Cited by
-
Outcomes in Adults With Acute Liver Failure Between 1998 and 2013: An Observational Cohort Study.Ann Intern Med. 2016 Jun 7;164(11):724-32. doi: 10.7326/M15-2211. Epub 2016 Apr 5. Ann Intern Med. 2016. PMID: 27043883 Free PMC article.
-
Acute liver failure following paracetamol overdose.J Intensive Care Soc. 2022 May;23(2):244-251. doi: 10.1177/17511437211007777. Epub 2021 Apr 15. J Intensive Care Soc. 2022. PMID: 35615243 Free PMC article.
-
Management of hepatic encephalopathy.Curr Treat Options Neurol. 2014 Jun;16(6):297. doi: 10.1007/s11940-014-0297-2. Curr Treat Options Neurol. 2014. PMID: 24807164
-
Intracranial pressure monitoring in the perioperative period of patients with acute liver failure undergoing orthotopic liver transplantation.World J Transplant. 2023 Jun 18;13(4):122-128. doi: 10.5500/wjt.v13.i4.122. World J Transplant. 2023. PMID: 37388394 Free PMC article. Review.
-
Optic Nerve Sheath Diameter in Acute Liver Failure: A Prospective Cohort Study.GE Port J Gastroenterol. 2021 Apr;28(3):170-178. doi: 10.1159/000511646. Epub 2020 Dec 2. GE Port J Gastroenterol. 2021. PMID: 34056039 Free PMC article.
References
-
- O’Grady JG, Williams R. Classification of acute liver failure. Lancet. 1993;342(8873):743. - PubMed
-
- Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42(6):1364–1372. - PubMed
-
- Ware AJ, D’Agostino AN, Combes B. Cerebral edema: a major complication of massive hepatic necrosis. Gastroenterology. 1971;61(6):877–884. - PubMed
-
- Bernal W, Wendon J. Acute liver failure; clinical features and management. Eur J Gastroenterol Hepatol. 1999;11(9):977–984. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

