RevSex duplication-induced and sex-related differences in the SOX9 regulatory region chromatin landscape in human fibroblasts
- PMID: 24351654
- PMCID: PMC4053460
- DOI: 10.4161/epi.27474
RevSex duplication-induced and sex-related differences in the SOX9 regulatory region chromatin landscape in human fibroblasts
Abstract
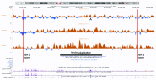
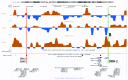
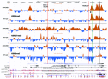
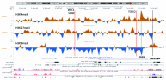
It was recently shown that duplications of the RevSex element, located 0.5 Mb upstream of SOX9, cause XX-disorder of sex development (DSD), and that deletions cause XY-DSD. To explore how a 148 kb RevSex duplication could have turned on gonadal SOX9 expression in the absence of SRY in an XX-male, we examined the chromatin landscape in primary skin fibroblast cultures from the index, his RevSex duplication-carrier father and six controls. The ENCODE project supports the notion that chromatin state maps show overlap between different cell types, i.e., that our study of fibroblasts could be of biological relevance. We examined the SOX9 regulatory region by high-resolution ChIP-on-chip experiments (a kind of "chromatin-CGH") and DNA methylation investigations. The RevSex duplication was associated with chromatin changes predicting better accessibility of the SRY-responsive TESCO enhancer region 14-15 kb upstream of SOX9. Four kb downstream of the TESCO evolutionary conserved region, a peak of the enhancer/promoter-associated H3K4me3 mark was found together with a major dip of the repressive H3K9me3 chromatin mark. Similar differences were also found when three control males were compared with three control females. A marked male/female difference was a more open chromatin signature in males starting ~400 kb upstream of SOX9 and increasing toward the SOX9 promoter. In the RevSex duplication-carrier father, two positions of DNA hypomethylation were also found, one corresponding to the H3K4me3 peak mentioned above. Our results suggest that the RevSex duplication could operate by inducing long-range epigenetic changes. Furthermore, the differences in chromatin state maps between males and females suggest that the Y chromosome or X chromosome dosage may affect chromatin conformation, i.e., that sex-dependent gene regulation may take place by chromatin modification.
Keywords: 46,XX DSD; ChIP-on-chip; RevSex duplication; SOX9; TESCO; chromatin; epigenetics; sex determination.
Figures

Similar articles
-
Disruption of a long distance regulatory region upstream of SOX9 in isolated disorders of sex development.J Med Genet. 2011 Dec;48(12):825-30. doi: 10.1136/jmedgenet-2011-100255. Epub 2011 Nov 2. J Med Genet. 2011. PMID: 22051515
-
Duplication of SOX9 associated with 46,XX ovotesticular disorder of sex development.Reprod Biomed Online. 2018 Jul;37(1):107-112. doi: 10.1016/j.rbmo.2018.03.017. Epub 2018 Apr 4. Reprod Biomed Online. 2018. PMID: 29673731
-
Copy number variation of two separate regulatory regions upstream of SOX9 causes isolated 46,XY or 46,XX disorder of sex development.J Med Genet. 2015 Apr;52(4):240-7. doi: 10.1136/jmedgenet-2014-102864. Epub 2015 Jan 20. J Med Genet. 2015. PMID: 25604083
-
The molecular action and regulation of the testis-determining factors, SRY (sex-determining region on the Y chromosome) and SOX9 [SRY-related high-mobility group (HMG) box 9].Endocr Rev. 2003 Aug;24(4):466-87. doi: 10.1210/er.2002-0025. Endocr Rev. 2003. PMID: 12920151 Review.
-
Testis determination in mammals: more questions than answers.Mol Cell Endocrinol. 2001 Jun 20;179(1-2):3-16. doi: 10.1016/s0303-7207(01)00460-9. Mol Cell Endocrinol. 2001. PMID: 11420125 Review.
Cited by
-
Loss of p300 and CBP disrupts histone acetylation at the mouse Sry promoter and causes XY gonadal sex reversal.Hum Mol Genet. 2018 Jan 1;27(1):190-198. doi: 10.1093/hmg/ddx398. Hum Mol Genet. 2018. PMID: 29145650 Free PMC article.
-
Testis development in the absence of SRY: chromosomal rearrangements at SOX9 and SOX3.Eur J Hum Genet. 2015 Aug;23(8):1025-32. doi: 10.1038/ejhg.2014.237. Epub 2014 Nov 5. Eur J Hum Genet. 2015. PMID: 25351776 Free PMC article.
-
The male and female genomes of golden pompano (Trachinotus ovatus) provide insights into the sex chromosome evolution and rapid growth.J Adv Res. 2024 Nov;65:1-17. doi: 10.1016/j.jare.2023.11.030. Epub 2023 Dec 2. J Adv Res. 2024. PMID: 38043610 Free PMC article.
-
XX Disorder of Sex Development is associated with an insertion on chromosome 9 and downregulation of RSPO1 in dogs (Canis lupus familiaris).PLoS One. 2017 Oct 20;12(10):e0186331. doi: 10.1371/journal.pone.0186331. eCollection 2017. PLoS One. 2017. PMID: 29053721 Free PMC article.
-
Diverse Regulation but Conserved Function: SOX9 in Vertebrate Sex Determination.Genes (Basel). 2021 Mar 26;12(4):486. doi: 10.3390/genes12040486. Genes (Basel). 2021. PMID: 33810596 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
