Evolution stings: the origin and diversification of scorpion toxin peptide scaffolds
- PMID: 24351712
- PMCID: PMC3873696
- DOI: 10.3390/toxins5122456
Evolution stings: the origin and diversification of scorpion toxin peptide scaffolds
Abstract
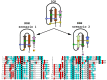
The episodic nature of natural selection and the accumulation of extreme sequence divergence in venom-encoding genes over long periods of evolutionary time can obscure the signature of positive Darwinian selection. Recognition of the true biocomplexity is further hampered by the limited taxon selection, with easy to obtain or medically important species typically being the subject of intense venom research, relative to the actual taxonomical diversity in nature. This holds true for scorpions, which are one of the most ancient terrestrial venomous animal lineages. The family Buthidae that includes all the medically significant species has been intensely investigated around the globe, while almost completely ignoring the remaining non-buthid families. Australian scorpion lineages, for instance, have been completely neglected, with only a single scorpion species (Urodacus yaschenkoi) having its venom transcriptome sequenced. Hence, the lack of venom composition and toxin sequence information from an entire continent's worth of scorpions has impeded our understanding of the molecular evolution of scorpion venom. The molecular origin, phylogenetic relationships and evolutionary histories of most scorpion toxin scaffolds remain enigmatic. In this study, we have sequenced venom gland transcriptomes of a wide taxonomical diversity of scorpions from Australia, including buthid and non-buthid representatives. Using state-of-art molecular evolutionary analyses, we show that a majority of CSα/β toxin scaffolds have experienced episodic influence of positive selection, while most non-CSα/β linear toxins evolve under the extreme influence of negative selection. For the first time, we have unraveled the molecular origin of the major scorpion toxin scaffolds, such as scorpion venom single von Willebrand factor C-domain peptides (SV-SVC), inhibitor cystine knot (ICK), disulphide-directed beta-hairpin (DDH), bradykinin potentiating peptides (BPP), linear non-disulphide bridged peptides and antimicrobial peptides (AMP). We have thus demonstrated that even neglected lineages of scorpions are a rich pool of novel biochemical components, which have evolved over millions of years to target specific ion channels in prey animals, and as a result, possess tremendous implications in therapeutics.
Figures




Similar articles
-
Molecular diversity of Chaerilidae venom peptides reveals the dynamic evolution of scorpion venom components from Buthidae to non-Buthidae.J Proteomics. 2013 Aug 26;89:1-14. doi: 10.1016/j.jprot.2013.06.007. Epub 2013 Jun 15. J Proteomics. 2013. PMID: 23774330
-
Functional evolution of scorpion venom peptides with an inhibitor cystine knot fold.Biosci Rep. 2013 Jun 27;33(3):e00047. doi: 10.1042/BSR20130052. Biosci Rep. 2013. PMID: 23721518 Free PMC article.
-
Whole Transcriptome of the Venom Gland from Urodacus yaschenkoi Scorpion.PLoS One. 2015 May 28;10(5):e0127883. doi: 10.1371/journal.pone.0127883. eCollection 2015. PLoS One. 2015. PMID: 26020943 Free PMC article.
-
Genetic mechanisms of scorpion venom peptide diversification.Toxicon. 2006 Mar;47(3):348-55. doi: 10.1016/j.toxicon.2005.11.013. Epub 2006 Jan 4. Toxicon. 2006. PMID: 16387337 Review.
-
An overview of toxins and genes from the venom of the Asian scorpion Buthus martensi Karsch.Toxicon. 2002 Sep;40(9):1239-58. doi: 10.1016/s0041-0101(02)00142-3. Toxicon. 2002. PMID: 12220709 Review.
Cited by
-
Molecular Diversity and Isoform Evolution in Tityus obscurus Venom: Insights from Proteomic Analysis.Toxins (Basel). 2025 Apr 23;17(5):210. doi: 10.3390/toxins17050210. Toxins (Basel). 2025. PMID: 40423293 Free PMC article.
-
Spider Venom: Components, Modes of Action, and Novel Strategies in Transcriptomic and Proteomic Analyses.Toxins (Basel). 2019 Oct 22;11(10):611. doi: 10.3390/toxins11100611. Toxins (Basel). 2019. PMID: 31652611 Free PMC article. Review.
-
A robust genome assembly with transcriptomic data from the striped bark scorpion, Centruroides vittatus.G3 (Bethesda). 2024 Aug 7;14(8):jkae120. doi: 10.1093/g3journal/jkae120. G3 (Bethesda). 2024. PMID: 38885085 Free PMC article.
-
Venom Peptides with Dual Modulatory Activity on the Voltage-Gated Sodium Channel NaV1.1 Provide Novel Leads for Development of Antiepileptic Drugs.ACS Pharmacol Transl Sci. 2019 Nov 25;3(1):119-134. doi: 10.1021/acsptsci.9b00079. eCollection 2020 Feb 14. ACS Pharmacol Transl Sci. 2019. PMID: 32259093 Free PMC article.
-
Tick Paralysis: Solving an Enigma.Vet Sci. 2018 May 14;5(2):53. doi: 10.3390/vetsci5020053. Vet Sci. 2018. PMID: 29757990 Free PMC article. Review.
References
-
- Brust A., Sunagar K., Undheim E.A.B., Vetter I., Yang D.C., Casewell N.R., Jackson T.N.W., Koludarov I., Alewood P.F., Hodgson W.C., Lewis R.J., King G.F., Antunes A., Hendrikx I., Fry B.G. Differential evolution and neofunctionalization of snake venom metalloprotease domains. Mol. Cell. Proteomics. 2013;12:651–663. doi: 10.1074/mcp.M112.023135. - DOI - PMC - PubMed
-
- Fry B.G., Scheib H., Weerd L. Van Der, Young B., Mcnaughtan J., Ramjan S.F.R., Vidal N., Poelmann R.E., Norman J.A. Evolution of an Arsenal. Mol. Cell. Proteomics. 2008:14–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

