Secreted phospholipases A2 of snake venoms: effects on the peripheral neuromuscular system with comments on the role of phospholipases A2 in disorders of the CNS and their uses in industry
- PMID: 24351716
- PMCID: PMC3873700
- DOI: 10.3390/toxins5122533
Secreted phospholipases A2 of snake venoms: effects on the peripheral neuromuscular system with comments on the role of phospholipases A2 in disorders of the CNS and their uses in industry
Abstract
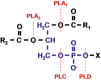
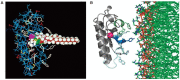
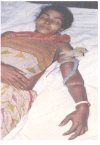
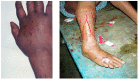
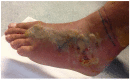
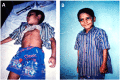
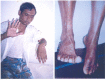
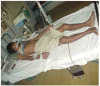
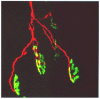
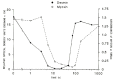
Neuro- and myotoxicological signs and symptoms are significant clinical features of envenoming snakebites in many parts of the world. The toxins primarily responsible for the neuro and myotoxicity fall into one of two categories--those that bind to and block the post-synaptic acetylcholine receptors (AChR) at the neuromuscular junction and neurotoxic phospholipases A2 (PLAs) that bind to and hydrolyse membrane phospholipids of the motor nerve terminal (and, in most cases, the plasma membrane of skeletal muscle) to cause degeneration of the nerve terminal and skeletal muscle. This review provides an introduction to the biochemical properties of secreted sPLA2s in the venoms of many dangerous snakes and a detailed discussion of their role in the initiation of the neurologically important consequences of snakebite. The rationale behind the experimental studies on the pharmacology and toxicology of the venoms and isolated PLAs in the venoms is discussed, with particular reference to the way these studies allow one to understand the biological basis of the clinical syndrome. The review also introduces the involvement of PLAs in inflammatory and degenerative disorders of the central nervous system (CNS) and their commercial use in the food industry. It concludes with an introduction to the problems associated with the use of antivenoms in the treatment of neuro-myotoxic snakebite and the search for alternative treatments.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

