Venom down under: dynamic evolution of Australian elapid snake toxins
- PMID: 24351719
- PMCID: PMC3873703
- DOI: 10.3390/toxins5122621
Venom down under: dynamic evolution of Australian elapid snake toxins
Abstract
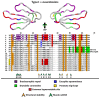
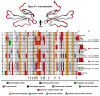
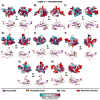
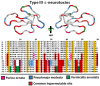
Despite the unparalleled diversity of venomous snakes in Australia, research has concentrated on a handful of medically significant species and even of these very few toxins have been fully sequenced. In this study, venom gland transcriptomes were sequenced from eleven species of small Australian elapid snakes, from eleven genera, spanning a broad phylogenetic range. The particularly large number of sequences obtained for three-finger toxin (3FTx) peptides allowed for robust reconstructions of their dynamic molecular evolutionary histories. We demonstrated that each species preferentially favoured different types of α-neurotoxic 3FTx, probably as a result of differing feeding ecologies. The three forms of α-neurotoxin [Type I (also known as (aka): short-chain), Type II (aka: long-chain) and Type III] not only adopted differential rates of evolution, but have also conserved a diversity of residues, presumably to potentiate prey-specific toxicity. Despite these differences, the different α-neurotoxin types were shown to accumulate mutations in similar regions of the protein, largely in the loops and structurally unimportant regions, highlighting the significant role of focal mutagenesis. We theorize that this phenomenon not only affects toxin potency or specificity, but also generates necessary variation for preventing/delaying prey animals from acquiring venom-resistance. This study also recovered the first full-length sequences for multimeric phospholipase A2 (PLA2) 'taipoxin/paradoxin' subunits from non-Oxyuranus species, confirming the early recruitment of this extremely potent neurotoxin complex to the venom arsenal of Australian elapid snakes. We also recovered the first natriuretic peptides from an elapid that lack the derived C-terminal tail and resemble the plesiotypic form (ancestral character state) found in viper venoms. This provides supporting evidence for a single early recruitment of natriuretic peptides into snake venoms. Novel forms of kunitz and waprin peptides were recovered, including dual domain kunitz-kunitz precursors and the first kunitz-waprin hybrid precursors from elapid snakes. The novel sequences recovered in this study reveal that the huge diversity of unstudied venomous Australian snakes are of considerable interest not only for the investigation of venom and whole organism evolution but also represent an untapped bioresource in the search for novel compounds for use in drug design and development.
Figures







Similar articles
-
Dynamic genetic differentiation drives the widespread structural and functional convergent evolution of snake venom proteinaceous toxins.BMC Biol. 2022 Jan 7;20(1):4. doi: 10.1186/s12915-021-01208-9. BMC Biol. 2022. PMID: 34996434 Free PMC article.
-
Venom gland transcriptomes of two elapid snakes (Bungarus multicinctus and Naja atra) and evolution of toxin genes.BMC Genomics. 2011 Jan 3;12:1. doi: 10.1186/1471-2164-12-1. BMC Genomics. 2011. PMID: 21194499 Free PMC article.
-
Clinical implications of convergent procoagulant toxicity and differential antivenom efficacy in Australian elapid snake venoms.Toxicol Lett. 2019 Nov;316:171-182. doi: 10.1016/j.toxlet.2019.08.014. Epub 2019 Aug 20. Toxicol Lett. 2019. PMID: 31442586
-
What Are the Neurotoxins in Hemotoxic Snake Venoms?Int J Mol Sci. 2023 Feb 2;24(3):2919. doi: 10.3390/ijms24032919. Int J Mol Sci. 2023. PMID: 36769242 Free PMC article. Review.
-
Studies on sea snake venom.Proc Jpn Acad Ser B Phys Biol Sci. 2011;87(3):41-52. doi: 10.2183/pjab.87.41. Proc Jpn Acad Ser B Phys Biol Sci. 2011. PMID: 21422738 Free PMC article. Review.
Cited by
-
Three-Finger Toxin Diversification in the Venoms of Cat-Eye Snakes (Colubridae: Boiga).J Mol Evol. 2018 Oct;86(8):531-545. doi: 10.1007/s00239-018-9864-6. Epub 2018 Sep 12. J Mol Evol. 2018. PMID: 30206667
-
Coagulating Colubrids: Evolutionary, Pathophysiological and Biodiscovery Implications of Venom Variations between Boomslang (Dispholidus typus) and Twig Snake (Thelotornis mossambicanus).Toxins (Basel). 2017 May 19;9(5):171. doi: 10.3390/toxins9050171. Toxins (Basel). 2017. PMID: 28534833 Free PMC article.
-
Activity of two key toxin groups in Australian elapid venoms show a strong correlation to phylogeny but not to diet.BMC Evol Biol. 2020 Jan 13;20(1):9. doi: 10.1186/s12862-020-1578-x. BMC Evol Biol. 2020. PMID: 31931699 Free PMC article.
-
Studying Venom Toxin Variation Using Accurate Masses from Liquid Chromatography-Mass Spectrometry Coupled with Bioinformatic Tools.Toxins (Basel). 2024 Apr 7;16(4):181. doi: 10.3390/toxins16040181. Toxins (Basel). 2024. PMID: 38668606 Free PMC article.
-
Coagulotoxicity of Bothrops (Lancehead Pit-Vipers) Venoms from Brazil: Differential Biochemistry and Antivenom Efficacy Resulting from Prey-Driven Venom Variation.Toxins (Basel). 2018 Oct 11;10(10):411. doi: 10.3390/toxins10100411. Toxins (Basel). 2018. PMID: 30314373 Free PMC article.
References
-
- Fry B.G., Roelants K., Champagne D.E., Scheib H., Tyndall J.D., King G.F., Nevalainen T.J., Norman J.A., Lewis R.J., Norton R.S., Renjifo C., de la Vega R.C. The toxicogenomic multiverse: convergent recruitment of proteins into animal venoms. Annu. Rev. Genomics Hum. Genet. 2009;10:483–511. doi: 10.1146/annurev.genom.9.081307.164356. - DOI - PubMed
-
- Earl S.T., Masci P.P., de Jersey J., Lavin M.F., Dixon J. Drug development from Australian elapid snake venoms and the Venomics pipeline of candidates for haemostasis: Textilinin-1 (Q8008), Haempatch (Q8009) and CoVase (V0801) Toxicon. 2012;59((4)):456–463. doi: 10.1016/j.toxicon.2010.12.010. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

