Increased pathogenicity of West Nile virus (WNV) by glycosylation of envelope protein and seroprevalence of WNV in wild birds in Far Eastern Russia
- PMID: 24351738
- PMCID: PMC3881158
- DOI: 10.3390/ijerph10127144
Increased pathogenicity of West Nile virus (WNV) by glycosylation of envelope protein and seroprevalence of WNV in wild birds in Far Eastern Russia
Abstract
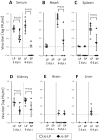
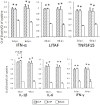
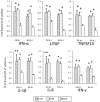
In this review, we discuss the possibility that the glycosylation of West Nile (WN) virus E-protein may be associated with enhanced pathogenicity and higher replication of WN virus. The results indicate that E-protein glycosylation allows the virus to multiply in a heat-stable manner and therefore, has a critical role in enhanced viremic levels and virulence of WN virus in young-chick infection model. The effect of the glycosylation of the E protein on the pathogenicity of WN virus in young chicks was further investigated. The results indicate that glycosylation of the WN virus E protein is important for viral multiplication in peripheral organs and that it is associated with the strong pathogenicity of WN virus in birds. The micro-focus reduction neutralization test (FRNT) in which a large number of serum samples can be handled at once with a small volume (15 μL) of serum was useful for differential diagnosis between Japanese encephalitis and WN virus infections in infected chicks. Serological investigation was performed among wild birds in the Far Eastern region of Russia using the FRNT. Antibodies specific to WN virus were detected in 21 samples of resident and migratory birds out of 145 wild bird samples in the region.
Figures


Similar articles
-
Seroprevalence of West Nile virus in wild birds in far eastern Russia using a focus reduction neutralization test.Am J Trop Med Hyg. 2011 Mar;84(3):461-5. doi: 10.4269/ajtmh.2011.09-0714. Am J Trop Med Hyg. 2011. PMID: 21363987 Free PMC article.
-
Glycosylation of the envelope protein of West Nile Virus affects its replication in chicks.Avian Dis. 2011 Dec;55(4):561-8. doi: 10.1637/9743-032811-Reg.1. Avian Dis. 2011. PMID: 22312974
-
Serological evidence of West Nile virus infection in wild migratory and resident water birds in Eastern and Northern India.Comp Immunol Microbiol Infect Dis. 2012 Dec;35(6):591-8. doi: 10.1016/j.cimid.2012.08.002. Epub 2012 Aug 25. Comp Immunol Microbiol Infect Dis. 2012. PMID: 22925932
-
The role of birds in the ecology of West Nile virus in Europe and Africa.Curr Top Microbiol Immunol. 2002;267:309-22. doi: 10.1007/978-3-642-59403-8_15. Curr Top Microbiol Immunol. 2002. PMID: 12082995 Review.
-
[West Nile virus. Prevalence and significance as a zoonotic pathogen].Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2004 Jul;47(7):653-60. doi: 10.1007/s00103-004-0864-x. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2004. PMID: 15254820 Review. German.
Cited by
-
Pathogenesis and Inhibition of Flaviviruses from a Carbohydrate Perspective.Pharmaceuticals (Basel). 2017 May 4;10(2):44. doi: 10.3390/ph10020044. Pharmaceuticals (Basel). 2017. PMID: 28471403 Free PMC article. Review.
-
An Overview of Current Approaches Toward the Treatment and Prevention of West Nile Virus Infection.Methods Mol Biol. 2016;1435:249-91. doi: 10.1007/978-1-4939-3670-0_19. Methods Mol Biol. 2016. PMID: 27188563 Free PMC article.
-
Evolution of Zika virus in Rag1-deficient mice selects for unique envelope glycosylation motif mutants that show enhanced replication fitness.Virus Evol. 2025 Apr 11;11(1):veaf021. doi: 10.1093/ve/veaf021. eCollection 2025. Virus Evol. 2025. PMID: 40291117 Free PMC article.
-
N-linked glycosylation of the West Nile virus envelope protein is not a requisite for avian virulence or vector competence.PLoS Negl Trop Dis. 2019 Jul 15;13(7):e0007473. doi: 10.1371/journal.pntd.0007473. eCollection 2019 Jul. PLoS Negl Trop Dis. 2019. PMID: 31306420 Free PMC article.
-
Structures and Functions of the Envelope Glycoprotein in Flavivirus Infections.Viruses. 2017 Nov 13;9(11):338. doi: 10.3390/v9110338. Viruses. 2017. PMID: 29137162 Free PMC article. Review.
References
-
- Hamman M.H., Delphine H.C., Winston H.P. Antigenic variation of West Nile virus in relation to geography. Amer. J. Epidemiol. 1965;82:40–55.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

