Oxidative stress and neurodegenerative disorders
- PMID: 24351827
- PMCID: PMC3876121
- DOI: 10.3390/ijms141224438
Oxidative stress and neurodegenerative disorders
Abstract
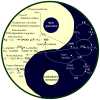
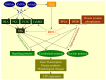
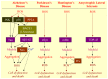
Living cells continually generate reactive oxygen species (ROS) through the respiratory chain during energetic metabolism. ROS at low or moderate concentration can play important physiological roles. However, an excessive amount of ROS under oxidative stress would be extremely deleterious. The central nervous system (CNS) is particularly vulnerable to oxidative stress due to its high oxygen consumption, weakly antioxidative systems and the terminal-differentiation characteristic of neurons. Thus, oxidative stress elicits various neurodegenerative diseases. In addition, chemotherapy could result in severe side effects on the CNS and peripheral nervous system (PNS) of cancer patients, and a growing body of evidence demonstrates the involvement of ROS in drug-induced neurotoxicities as well. Therefore, development of antioxidants as neuroprotective drugs is a potentially beneficial strategy for clinical therapy. In this review, we summarize the source, balance maintenance and physiologic functions of ROS, oxidative stress and its toxic mechanisms underlying a number of neurodegenerative diseases, and the possible involvement of ROS in chemotherapy-induced toxicity to the CNS and PNS. We ultimately assess the value for antioxidants as neuroprotective drugs and provide our comments on the unmet needs.
Figures

References
-
- Coon M.J., Ding X., Pernecky S.J., Vaz A.D.N. Cytochrome P450: Progress and predictions. FASEB J. 1992;6:669–673. - PubMed
-
- DeLeo F.R., Quinn M.T. Assembly of the phagocyte NADPH oxidase: Molecular interaction of oxidase proteins. J. Leukoc. Biol. 1996;60:677–691. - PubMed
-
- Finkel T. Redox-dependent signal transduction. FEBS Lett. 2000;476:52–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

