Vascular aldosterone production at the pre-diabetic stage of young Otsuka Long-Evans Tokushima Fatty (OLETF) rats, compared with Long-Evans Tokushima Otsuka (LETO) rats
- PMID: 24352019
- PMCID: PMC6270161
- DOI: 10.3390/molecules181215636
Vascular aldosterone production at the pre-diabetic stage of young Otsuka Long-Evans Tokushima Fatty (OLETF) rats, compared with Long-Evans Tokushima Otsuka (LETO) rats
Abstract
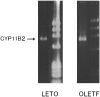
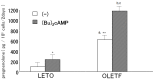
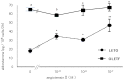
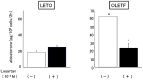
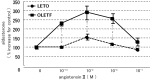
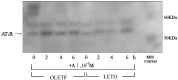
We examined the ability of aortic smooth muscle cells (AoSMC) prepared from spontaneously diabetic rats to produce aldosterone (Aldo) and the regulatory mechanism that controls their Aldo production. AoSMC of 6 week-old Long-Evans Tokushima Otsuka (LETO: the control group) and 6 week-old Otsuka Long-Evans Tokushima Fatty (OLETF: the type 2 diabetes group) rats were used in the present experiments. CYP11B2 (Aldo synthetase) mRNA expression was detected in both the LETO and OLETF AoSMC. Basal Aldo production was significantly greater (4-5 fold higher) in the OLETF AoSMC culture medium than in the LETO AoSMC culture medium. When AoSMC were co-incubated with high-density lipoproteins (HDL), supplying cholesterol as a substrate for steroidogenesis in rats, angiotensin II (AII) significantly increased greater Aldo production in the OLETF AoSMC than in the LETO AoSMC. The present data suggested that future onset of diabetic vascular dysfunction is partly caused by excess Aldo production by AoSMC in young OLETF rats. Concomitant stimulation by HDL and AII resulted in elevated Aldo production in the OLETF and the LETO AoSMC, and also demonstrated that AII-induced Aldo production is greatly enhanced by HDL in OLETF, rather than in LETO. In conclusion, our data clearly demonstrated that Aldo production in the OLETF AoSMC was significantly higher than in the LETO AoSMC, suggesting possible future onset of vascular dysfunction in diabetes, induced by local Aldo production in the AoSMC.
Figures

Similar articles
-
Vascular proliferation and transforming growth factor-beta expression in pre- and early stage of diabetes mellitus in Otsuka Long-Evans Tokushima fatty rats.Atherosclerosis. 2002 May;162(1):69-76. doi: 10.1016/s0021-9150(01)00683-9. Atherosclerosis. 2002. PMID: 11947899
-
Ultrastructural changes of cornea after ethanol ingestion in Otsuka Long-Evans Tokushima fatty (OLETF) and Long-Evans Tokushima Otsuka (LETO) rats.Graefes Arch Clin Exp Ophthalmol. 2010 Oct;248(10):1457-66. doi: 10.1007/s00417-010-1432-8. Epub 2010 Jun 27. Graefes Arch Clin Exp Ophthalmol. 2010. PMID: 20582705
-
Increased hepatic glucose production and decreased hepatic glucose uptake at the prediabetic phase in the Otsuka Long-Evans Tokushima fatty rat model.Metabolism. 1998 Aug;47(8):908-14. doi: 10.1016/s0026-0495(98)90343-2. Metabolism. 1998. PMID: 9711984
-
Increased sensitivity of serotonin on the voltage-dependent K+ channels in mesenteric arterial smooth muscle cells of OLETF rats.Prog Biophys Mol Biol. 2010 Sep;103(1):88-94. doi: 10.1016/j.pbiomolbio.2010.02.003. Epub 2010 Feb 26. Prog Biophys Mol Biol. 2010. PMID: 20219524 Review.
-
Therapeutic effects of sericin on diabetic keratopathy in Otsuka Long-Evans Tokushima Fatty rats.World J Diabetes. 2013 Dec 15;4(6):282-9. doi: 10.4239/wjd.v4.i6.282. World J Diabetes. 2013. PMID: 24379918 Free PMC article. Review.
Cited by
-
No extra-adrenal aldosterone production in various human cell lines.J Mol Endocrinol. 2024 Feb 1;72(3):e230100. doi: 10.1530/JME-23-0100. Print 2024 Apr 1. J Mol Endocrinol. 2024. PMID: 38175924 Free PMC article.
-
Therapeutic Interference With Vascular Calcification-Lessons From Klotho-Hypomorphic Mice and Beyond.Front Endocrinol (Lausanne). 2018 May 4;9:207. doi: 10.3389/fendo.2018.00207. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29780355 Free PMC article. Review.
-
Local aldosterone synthesis in the large intestine of mouse: An ex vivo incubation study.J Int Med Res. 2022 Jun;50(6):3000605221105163. doi: 10.1177/03000605221105163. J Int Med Res. 2022. PMID: 35748030 Free PMC article.
-
Estrogen biology: new insights into GPER function and clinical opportunities.Mol Cell Endocrinol. 2014 May 25;389(1-2):71-83. doi: 10.1016/j.mce.2014.02.002. Epub 2014 Feb 12. Mol Cell Endocrinol. 2014. PMID: 24530924 Free PMC article. Review.
-
Endothelial Dysfunction and Passive Changes in the Aorta and Coronary Arteries of Diabetic db/db Mice.Front Physiol. 2020 Jun 23;11:667. doi: 10.3389/fphys.2020.00667. eCollection 2020. Front Physiol. 2020. PMID: 32655412 Free PMC article.
References
-
- Hatakeyama H., Miyamori I., Takeda Y., Yamamoto H., Mabuchi H. The expression of steroidogenic enzyme genes in human vascular cells. Biochem. Mol. Biol. Int. 1996;40:639–645. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

