A three-dimensional atlas of human dermal leukocytes, lymphatics, and blood vessels
- PMID: 24352044
- PMCID: PMC3961477
- DOI: 10.1038/jid.2013.481
A three-dimensional atlas of human dermal leukocytes, lymphatics, and blood vessels
Abstract
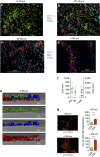
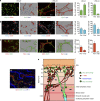
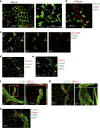
Dendritic cells (DCs), macrophages (Mφ), and T cells are major components of the skin immune system, but their interstitial spatial organization is poorly characterized. Using four-channel whole-mount immunofluorescence staining of the human dermis, we demonstrated the three-dimensional distribution of CD31(+) blood capillaries, LYVE-1(+) lymphatics, discrete populations of CD11c(+) myeloid DCs, FXIIIa(+) Mφ, and lymphocytes. We showed phenotypic and morphological differences in situ between DCs and Mφ. DCs formed the first dermal cellular layer (0-20 μm beneath the dermoepidermal junction), Mφ were located deeper (40-60 μm), and CD3(+) lymphocytes were observed throughout (0-60 μm). Below this level, DCs, T cells, and the majority of Mφ formed stable perivascular sheaths. Whole-mount imaging revealed the true extent of dermal leukocytes previously underestimated from cross-section views. The total area of apical dermis (0-30 μm) contained approximately 10-fold more myeloid DCs than the entire blood volume of an average individual. Surprisingly, <1% of dermal DCs occupied lymphatics in freshly isolated skin. Dermal DCs rapidly accumulated within lymphatics, but Mφ remained fixed in skin explants cultured ex vivo. The leukocyte architecture observed in normal skin was distorted in inflammation and disease. These studies illustrate the micro-anatomy of dermal leukocytes and provide further insights into their functional organization.
Figures



References
-
- Bockle BC, Solder E, Kind S, et al. DC-sign+ CD163+ macrophages expressing hyaluronan receptor LYVE-1 are located within chorion villi of the placenta. Placenta. 2008;29:187–192. - PubMed
-
- Brand CU, Hunger RE, Yawalkar N, et al. Characterization of human skin-derived CD1a-positive lymph cells. Arch Dermatol Res. 1999;291:65–72. - PubMed
-
- Cerio R, Griffiths CE, Cooper KD, et al. Characterization of factor XIIIa positive dermal dendritic cells in normal and inflamed skin. Br J Dermatol. 1989;121:421–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

