Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels
- PMID: 24352100
- PMCID: PMC6506564
- DOI: 10.1038/srep03553
Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels
Abstract
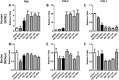
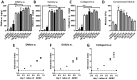
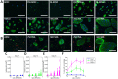
Cartilage loss is a leading cause of disability among adults and effective therapy remains elusive. Neonatal chondrocytes (NChons) are an attractive allogeneic cell source for cartilage repair, but their clinical translation has been hindered by scarce donor availability. Here we examine the potential for catalyzing cartilage tissue formation using a minimal number of NChons by co-culturing them with adipose-derived stem cells (ADSCs) in 3D hydrogels. Using three different co-culture models, we demonstrated that the effects of co-culture on cartilage tissue formation are dependent on the intercellular distance and cell distribution in 3D. Unexpectedly, increasing ADSC ratio in mixed co-culture led to increased synergy between NChons and ADSCs, and resulted in the formation of large neocartilage nodules. This work raises the potential of utilizing stem cells to catalyze tissue formation by neonatal chondrocytes via paracrine signaling, and highlights the importance of controlling cell distribution in 3D matrices to achieve optimal synergy.
Conflict of interest statement
This work has been disclosed to the Office Technology Licensing at Stanford University.
Figures
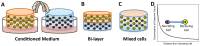

Similar articles
-
Comparative potential of juvenile and adult human articular chondrocytes for cartilage tissue formation in three-dimensional biomimetic hydrogels.Tissue Eng Part A. 2015 Jan;21(1-2):147-55. doi: 10.1089/ten.TEA.2014.0070. Epub 2014 Oct 1. Tissue Eng Part A. 2015. PMID: 25054343
-
Microribbon-hydrogel composite scaffold accelerates cartilage regeneration in vivo with enhanced mechanical properties using mixed stem cells and chondrocytes.Biomaterials. 2020 Jan;228:119579. doi: 10.1016/j.biomaterials.2019.119579. Epub 2019 Oct 31. Biomaterials. 2020. PMID: 31698227
-
Effects of Hydrogel Stiffness and Extracellular Compositions on Modulating Cartilage Regeneration by Mixed Populations of Stem Cells and Chondrocytes In Vivo.Tissue Eng Part A. 2016 Dec;22(23-24):1348-1356. doi: 10.1089/ten.TEA.2016.0306. Epub 2016 Oct 19. Tissue Eng Part A. 2016. PMID: 27676200 Free PMC article.
-
The Challenge in Using Mesenchymal Stromal Cells for Recellularization of Decellularized Cartilage.Stem Cell Rev Rep. 2017 Feb;13(1):50-67. doi: 10.1007/s12015-016-9699-8. Stem Cell Rev Rep. 2017. PMID: 27826794 Review.
-
Cartilage Tissue Regeneration: The Roles of Cells, Stimulating Factors and Scaffolds.Curr Stem Cell Res Ther. 2018;13(7):547-567. doi: 10.2174/1574888X12666170608080722. Curr Stem Cell Res Ther. 2018. PMID: 28595567 Review.
Cited by
-
Maintenance and Acceleration of Pericellular Matrix Formation within 3D Cartilage Cell Culture Models.Cartilage. 2021 Dec;13(2_suppl):847S-861S. doi: 10.1177/1947603519870839. Epub 2019 Aug 28. Cartilage. 2021. PMID: 31455088 Free PMC article.
-
Induced pluripotent stem cells in cartilage repair.World J Orthop. 2016 Mar 18;7(3):149-55. doi: 10.5312/wjo.v7.i3.149. eCollection 2016 Mar 18. World J Orthop. 2016. PMID: 27004161 Free PMC article.
-
Mechanical confinement regulates cartilage matrix formation by chondrocytes.Nat Mater. 2017 Dec;16(12):1243-1251. doi: 10.1038/nmat4993. Epub 2017 Oct 2. Nat Mater. 2017. PMID: 28967913 Free PMC article.
-
Modulation of Hyaluronan Synthesis by the Interaction between Mesenchymal Stem Cells and Osteoarthritic Chondrocytes.Stem Cells Int. 2015;2015:640218. doi: 10.1155/2015/640218. Epub 2015 Jul 26. Stem Cells Int. 2015. PMID: 26273306 Free PMC article.
-
hBMSC-Derived Extracellular Vesicles Attenuate IL-1β-Induced Catabolic Effects on OA-Chondrocytes by Regulating Pro-inflammatory Signaling Pathways.Front Bioeng Biotechnol. 2020 Dec 14;8:603598. doi: 10.3389/fbioe.2020.603598. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33425869 Free PMC article.
References
-
- Griffin T. M. & Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev 33, 195–200 (2005). - PubMed
-
- Brittberg M. et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331, 889–95 (1994). - PubMed
-
- Saha S., Kirkham J., Wood D., Curran S. & Yang X. Comparative study of the chondrogenic potential of human bone marrow stromal cells, neonatal chondrocytes and adult chondrocytes. Biochem Biophys Res Commun 401, 333–8 (2010). - PubMed
-
- Adkisson H. D., Gillis M. P., Davis E. C., Maloney W. & Hruska K. A. In vitro generation of scaffold independent neocartilage. Clin Orthop Relat Res, S280–94 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

