Hallmarks of a new era in mitochondrial biochemistry
- PMID: 24352419
- PMCID: PMC3877752
- DOI: 10.1101/gad.229724.113
Hallmarks of a new era in mitochondrial biochemistry
Abstract
Stemming from the pioneering studies of bioenergetics in the 1950s, 1960s, and 1970s, mitochondria have become ingrained in the collective psyche of scientists as the "powerhouses" of the cell. While this remains a worthy moniker, more recent efforts have revealed that these organelles are home to a vast array of metabolic and signaling processes and possess a proteomic landscape that is both highly varied and largely uncharted. As mitochondrial dysfunction is increasingly being implicated in a spectrum of human diseases, it is imperative that we construct a more complete framework of these organelles by systematically defining the functions of their component parts. Powerful new approaches in biochemistry and systems biology are helping to fill in the gaps.
Keywords: biochemistry; mitochondria; proteomics.
Figures
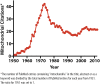

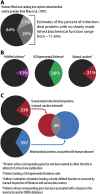
References
-
- Alam MR, Groschner LN, Parichatikanond W, Kuo L, Bondarenko AI, Rost R, Waldeck-Weiermair M, Malli R, Graier WF 2012. Mitochondrial Ca2+ uptake 1 (MICU1) and mitochondrial Ca2+ uniporter (MCU) contribute to metabolism–secretion coupling in clonal pancreatic β-cells. J Biol Chem 287: 34445–34454 - PMC - PubMed
-
- Altmann R. 1890. Die Elementarorganismen und ihre beziehungen zu den zellen. Veit, Leipzig, Germany.
-
- Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, et al. 1981. Sequence and organization of the human mitochondrial genome. Nature 290: 457–465 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
