eIF2B promotes eIF5 dissociation from eIF2*GDP to facilitate guanine nucleotide exchange for translation initiation
- PMID: 24352424
- PMCID: PMC3877758
- DOI: 10.1101/gad.231514.113
eIF2B promotes eIF5 dissociation from eIF2*GDP to facilitate guanine nucleotide exchange for translation initiation
Abstract
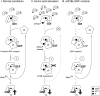
Protein synthesis factor eIF2 delivers initiator tRNA to the ribosome. Two proteins regulate its G-protein cycle: eIF5 has both GTPase-accelerating protein (GAP) and GDP dissociation inhibitor (GDI) functions, and eIF2B is the guanine nucleotide exchange factor (GEF). In this study, we used protein-protein interaction and nucleotide exchange assays to monitor the kinetics of eIF2 release from the eIF2•GDP/eIF5 GDI complex and determine the effect of eIF2B on this release. We demonstrate that eIF2B has a second activity as a GDI displacement factor (GDF) that can recruit eIF2 from the eIF2•GDP/eIF5 GDI complex prior to GEF action. We found that GDF function is dependent on the eIF2Bε and eIF2Bγ subunits and identified a novel eIF2-eIF2Bγ interaction. Furthermore, GDF and GEF activities are shown to be independent. First, eIF2B GDF is insensitive to eIF2α phosphorylation, unlike GEF. Second, we found that eIF2Bγ mutations known to disrupt GCN4 translational control significantly impair GDF activity but not GEF function. Our data therefore define an additional step in the protein synthesis initiation pathway that is important for its proper control. We propose a new model to place eIF2B GDF function in the context of efficient eIF2 recycling and its regulation by eIF2 phosphorylation.
Keywords: G-protein regulators; GDF; GDI; GEF; protein synthesis initiation.
Figures





References
-
- Algire MA, Maag D, Lorsch JR 2005. Pi release from eIF2, not GTP hydrolysis, is the step controlled by start-site selection during eukaryotic translation initiation. Mol Cell 20: 251–262 - PubMed
-
- Alone PV, Dever TE 2006. Direct binding of translation initiation factor eIF2γ-G domain to its GTPase-activating and GDP–GTP exchange factors eIF5 and eIF2Bɛ. J Biol Chem 281: 12636–12644 - PubMed
-
- Amberg DC, Burke D, Strathern JN, Laboratory CSH 2005. Methods in yeast genetics: A Cold Spring Harbor Laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
