Recruitment of PI4KIIIβ to coxsackievirus B3 replication organelles is independent of ACBD3, GBF1, and Arf1
- PMID: 24352456
- PMCID: PMC3958084
- DOI: 10.1128/JVI.03650-13
Recruitment of PI4KIIIβ to coxsackievirus B3 replication organelles is independent of ACBD3, GBF1, and Arf1
Abstract
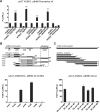
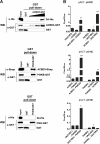
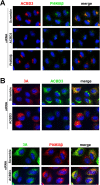
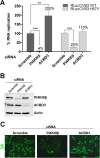
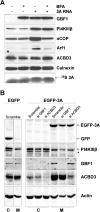
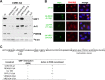
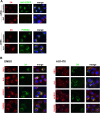
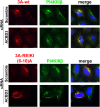
Members of the Enterovirus (poliovirus [PV], coxsackieviruses, and human rhinoviruses) and Kobuvirus (Aichi virus) genera in the Picornaviridae family rely on PI4KIIIβ (phosphatidylinositol-4-kinase IIIβ) for efficient replication. The small membrane-anchored enteroviral protein 3A recruits PI4KIIIβ to replication organelles, yet the underlying mechanism has remained elusive. Recently, it was shown that kobuviruses recruit PI4KIIIβ through interaction with ACBD3 (acyl coenzyme A [acyl-CoA]-binding protein domain 3), a novel interaction partner of PI4KIIIβ. Therefore, we investigated a possible role for ACBD3 in recruiting PI4KIIIβ to enterovirus replication organelles. Although ACBD3 interacted directly with coxsackievirus B3 (CVB3) 3A, its depletion from cells by RNA interference did not affect PI4KIIIβ recruitment to replication organelles and did not impair CVB3 RNA replication. Enterovirus 3A was previously also proposed to recruit PI4KIIIβ via GBF1/Arf1, based on the known interaction of 3A with GBF1, an important regulator of secretory pathway transport and a guanine nucleotide exchange factor (GEF) of Arf1. However, our results demonstrate that inhibition of GBF1 or Arf1 either by pharmacological inhibition or depletion with small interfering RNA (siRNA) treatment did not affect the ability of 3A to recruit PI4KIIIβ. Furthermore, we show that a 3A mutant that no longer binds GBF1 was capable of recruiting PI4KIIIβ, even in ACBD3-depleted cells. Together, our findings indicate that unlike originally envisaged, coxsackievirus recruits PI4KIIIβ to replication organelles independently of ACBD3 and GBF1/Arf1.
Importance: A hallmark of enteroviral infection is the generation of new membranous structures to support viral RNA replication. The functionality of these "replication organelles" depends on the concerted actions of both viral nonstructural proteins and co-opted host factors. It is thus essential to understand how these structures are formed and which cellular components are key players in this process. GBF1/Arf1 and ACBD3 have been proposed to contribute to the recruitment of the essential lipid-modifying enzyme PI4KIIIβ to enterovirus replication organelles. Here we show that the enterovirus CVB3 recruits PI4KIIIβ by a mechanism independent of both GBF1/Arf1 and ACBD3. This study shows that the strategy employed by coxsackievirus to recruit PI4KIIIβ to replication organelles is far more complex than initially anticipated.
Figures
Similar articles
-
ACBD3 Is an Essential Pan-enterovirus Host Factor That Mediates the Interaction between Viral 3A Protein and Cellular Protein PI4KB.mBio. 2019 Feb 12;10(1):e02742-18. doi: 10.1128/mBio.02742-18. mBio. 2019. PMID: 30755512 Free PMC article.
-
GBF1- and ACBD3-independent recruitment of PI4KIIIβ to replication sites by rhinovirus 3A proteins.J Virol. 2015 Feb;89(3):1913-8. doi: 10.1128/JVI.02830-14. Epub 2014 Nov 19. J Virol. 2015. PMID: 25410869 Free PMC article.
-
The 3A protein from multiple picornaviruses utilizes the golgi adaptor protein ACBD3 to recruit PI4KIIIβ.J Virol. 2012 Apr;86(7):3605-16. doi: 10.1128/JVI.06778-11. Epub 2012 Jan 18. J Virol. 2012. PMID: 22258260 Free PMC article.
-
Emerging Role for Acyl-CoA Binding Domain Containing 3 at Membrane Contact Sites During Viral Infection.Front Microbiol. 2020 Apr 8;11:608. doi: 10.3389/fmicb.2020.00608. eCollection 2020. Front Microbiol. 2020. PMID: 32322249 Free PMC article. Review.
-
The role of phosphatidylinositol 4-kinases and phosphatidylinositol 4-phosphate during viral replication.Biochem Pharmacol. 2012 Dec 1;84(11):1400-8. doi: 10.1016/j.bcp.2012.07.034. Epub 2012 Aug 8. Biochem Pharmacol. 2012. PMID: 22885339 Free PMC article. Review.
Cited by
-
Curcumol inhibits EMCV replication by activating CH25H and inhibiting the formation of ROs.BMC Vet Res. 2022 Dec 26;18(1):453. doi: 10.1186/s12917-022-03531-x. BMC Vet Res. 2022. PMID: 36572890 Free PMC article.
-
CRISPR-Cas9-Based Technology for Studying Enteric Virus Infection.Front Genome Ed. 2022 Jun 8;4:888878. doi: 10.3389/fgeed.2022.888878. eCollection 2022. Front Genome Ed. 2022. PMID: 35755450 Free PMC article. Review.
-
ACBD3 Is an Essential Pan-enterovirus Host Factor That Mediates the Interaction between Viral 3A Protein and Cellular Protein PI4KB.mBio. 2019 Feb 12;10(1):e02742-18. doi: 10.1128/mBio.02742-18. mBio. 2019. PMID: 30755512 Free PMC article.
-
Enterovirus 3A Facilitates Viral Replication by Promoting Phosphatidylinositol 4-Kinase IIIβ-ACBD3 Interaction.J Virol. 2017 Sep 12;91(19):e00791-17. doi: 10.1128/JVI.00791-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28701404 Free PMC article.
-
A Crucial Role of ACBD3 Required for Coxsackievirus Infection in Animal Model Developed by AAV-Mediated CRISPR Genome Editing Technique.Viruses. 2021 Feb 3;13(2):237. doi: 10.3390/v13020237. Viruses. 2021. PMID: 33546322 Free PMC article.
References
-
- Romero-Brey I, Merz A, Chiramel A, Lee J-Y, Chlanda P, Haselman U, Santarella-Mellwig R, Habermann A, Hoppe S, Kallis S, Walther P, Antony C, Krijnse-Locker J, Bartenschlager R. 2012. Three-dimensional architecture and biogenesis of membrane structures associated with hepatitis C virus replication. PLoS Pathog. 8:e1003056. 10.1371/journal.ppat.1003056 - DOI - PMC - PubMed
-
- Welsch S, Miller S, Romero-Brey I, Merz A, Bleck CKE, Walther P, Fuller SD, Antony C, Krijnse-Locker J, Bartenschlager R. 2009. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe 5:365–375. 10.1016/j.chom.2009.03.007 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

