Kaposi's sarcoma-associated herpesvirus induces the ATM and H2AX DNA damage response early during de novo infection of primary endothelial cells, which play roles in latency establishment
- PMID: 24352470
- PMCID: PMC3958070
- DOI: 10.1128/JVI.03126-13
Kaposi's sarcoma-associated herpesvirus induces the ATM and H2AX DNA damage response early during de novo infection of primary endothelial cells, which play roles in latency establishment
Abstract
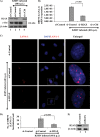
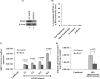
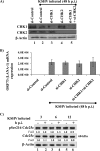
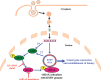
The DNA damage response (DDR) that evolved to repair host cell DNA damage also recognizes viral DNA entering the nucleus during infections. Here, we investigated the modulation of DDR signaling during de novo infection of primary endothelial cells by Kaposi's sarcoma-associated herpesvirus (KSHV). Phosphorylation of representative DDR-associated proteins, such as ataxia telangiectasia mutated (ATM) and H2AX, was induced as early as 30 min (0.5 h) postinfection and persisted during in vitro KSHV latency. Phosphorylated H2AX (γH2AX) colocalized at 30 min (0.5 h) with the KSHV genome entering the nuclei. Total H2AX protein levels also increased, and the increase was attributed to a decrease in degradative H2AX Lys48-linked polyubiquitination with a concomitant increase in Lys63-linked polyubiquitination that was shown to increase protein stability. ATM and H2AX phosphorylation and γH2AX nuclear foci were also induced by UV-inactivated KSHV, which ceased at later times of infection. Inhibition of ATM kinase activity by KU-55933 and H2AX knockdown by small interfering RNA significantly reduced the expression of the KSHV latency-associated nuclear antigen 1 (LANA-1; ORF73) and LANA-1 nuclear puncta. Knockdown of H2AX also resulted in a >80% reduction in the nuclear KSHV DNA copy numbers. Similar results were also observed in ATM-negative cells, although comparable levels of viral DNA entered ATM-negative and ATM-positive cell nuclei. In contrast, knockdown of CHK1 and CHK2 did not affect ORF73 expression. Collectively, these results demonstrate that KSHV induces ATM and H2AX, a selective arm of the DDR, for the establishment and maintenance of its latency during de novo infection of primary endothelial cells.
Importance: Eukaryotic cells mount a DNA damage response (DDR) to sense and repair different types of cellular DNA damage. In addition, DDR also recognizes exogenous genetic material, such as the viral DNA genome entering the nucleus during infections. The present study was undertaken to determine whether de novo Kaposi's sarcoma-associated herpesvirus (KSHV) infection modulates DDR. Our results demonstrate that early during de novo infection of primary endothelial cells, KSHV induces a selective arm of DDR signaling, such as the ATM kinase and its downstream target, H2AX, which are essential for KSHV's latent gene expression and the establishment of latency. These studies suggest that targeting ATM and H2AX could serve as an attractive strategy to block the establishment of KSHV latent infection and the associated malignancies.
Figures


Similar articles
-
Activation of DNA Damage Response Induced by the Kaposi's Sarcoma-Associated Herpes Virus.Int J Mol Sci. 2016 Jun 1;17(6):854. doi: 10.3390/ijms17060854. Int J Mol Sci. 2016. PMID: 27258263 Free PMC article. Review.
-
H2AX phosphorylation is important for LANA-mediated Kaposi's sarcoma-associated herpesvirus episome persistence.J Virol. 2013 May;87(9):5255-69. doi: 10.1128/JVI.03575-12. Epub 2013 Feb 28. J Virol. 2013. PMID: 23449797 Free PMC article.
-
Localization of Double-Strand Break Repair Proteins to Viral Replication Compartments following Lytic Reactivation of Kaposi's Sarcoma-Associated Herpesvirus.J Virol. 2017 Oct 27;91(22):e00930-17. doi: 10.1128/JVI.00930-17. Print 2017 Nov 15. J Virol. 2017. PMID: 28855246 Free PMC article.
-
Transcriptome analysis of Kaposi's sarcoma-associated herpesvirus during de novo primary infection of human B and endothelial cells.J Virol. 2015 Mar;89(6):3093-111. doi: 10.1128/JVI.02507-14. Epub 2014 Dec 31. J Virol. 2015. PMID: 25552714 Free PMC article.
-
KSHV Genome Replication and Maintenance in Latency.Adv Exp Med Biol. 2018;1045:299-320. doi: 10.1007/978-981-10-7230-7_14. Adv Exp Med Biol. 2018. PMID: 29896673 Review.
Cited by
-
Modulation of DNA damage and repair pathways by human tumour viruses.Viruses. 2015 May 22;7(5):2542-91. doi: 10.3390/v7052542. Viruses. 2015. PMID: 26008701 Free PMC article. Review.
-
Ataxia Telangiectasia-Mutated Is Activated but Not Required for Productive Autographa californica Multiple Nucleopolyhedrovirus Infection.J Virol. 2022 Nov 23;96(22):e0126922. doi: 10.1128/jvi.01269-22. Epub 2022 Oct 31. J Virol. 2022. PMID: 36314821 Free PMC article.
-
Activation of DNA Damage Response Induced by the Kaposi's Sarcoma-Associated Herpes Virus.Int J Mol Sci. 2016 Jun 1;17(6):854. doi: 10.3390/ijms17060854. Int J Mol Sci. 2016. PMID: 27258263 Free PMC article. Review.
-
Oncolytic strategy using new bifunctional HDACs/BRD4 inhibitors against virus-associated lymphomas.PLoS Pathog. 2023 Jan 13;19(1):e1011089. doi: 10.1371/journal.ppat.1011089. eCollection 2023 Jan. PLoS Pathog. 2023. PMID: 36638143 Free PMC article.
-
Chk1 and the Host Cell DNA Damage Response as a Potential Antiviral Target in BK Polyomavirus Infection.Viruses. 2021 Jul 13;13(7):1353. doi: 10.3390/v13071353. Viruses. 2021. PMID: 34372559 Free PMC article.
References
-
- Ganem D. 2007. Kaposi's sarcoma-associated herpesvirus, p 2847–2888 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

