EGFRvIII mCAR-modified T-cell therapy cures mice with established intracerebral glioma and generates host immunity against tumor-antigen loss
- PMID: 24352643
- PMCID: PMC3943170
- DOI: 10.1158/1078-0432.CCR-13-0709
EGFRvIII mCAR-modified T-cell therapy cures mice with established intracerebral glioma and generates host immunity against tumor-antigen loss
Abstract
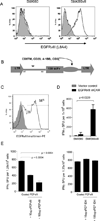
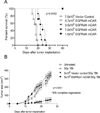
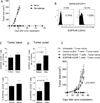
Purpose: Chimeric antigen receptor (CAR) transduced T cells represent a promising immune therapy that has been shown to successfully treat cancers in mice and humans. However, CARs targeting antigens expressed in both tumors and normal tissues have led to significant toxicity. Preclinical studies have been limited by the use of xenograft models that do not adequately recapitulate the immune system of a clinically relevant host. A constitutively activated mutant of the naturally occurring epidermal growth factor receptor (EGFRvIII) is antigenically identical in both human and mouse glioma, but is also completely absent from any normal tissues.
Experimental design: We developed a third-generation, EGFRvIII-specific murine CAR (mCAR), and performed tests to determine its efficacy in a fully immunocompetent mouse model of malignant glioma.
Results: At elevated doses, infusion with EGFRvIII mCAR T cells led to cures in all mice with brain tumors. In addition, antitumor efficacy was found to be dependent on lymphodepletive host conditioning. Selective blockade with EGFRvIII soluble peptide significantly abrogated the activity of EGFRvIII mCAR T cells in vitro and in vivo, and may offer a novel strategy to enhance the safety profile for CAR-based therapy. Finally, mCAR-treated, cured mice were resistant to rechallenge with EGFRvIII(NEG) tumors, suggesting generation of host immunity against additional tumor antigens.
Conclusion: All together, these data support that third-generation, EGFRvIII-specific mCARs are effective against gliomas in the brain and highlight the importance of syngeneic, immunocompetent models in the preclinical evaluation of tumor immunotherapies.
©2013 AACR
Conflict of interest statement
Disclosure statement: JHS, BDC and LAJ are named in an intellectual property filing by Duke University based upon the use of EGFRvIII peptide to abrogate CAR T-cell function in vivo. There are no other potential conflicts of interest to declare.
Figures


Similar articles
-
EGFRvIII-specific chimeric antigen receptor T cells migrate to and kill tumor deposits infiltrating the brain parenchyma in an invasive xenograft model of glioblastoma.PLoS One. 2014 Apr 10;9(4):e94281. doi: 10.1371/journal.pone.0094281. eCollection 2014. PLoS One. 2014. PMID: 24722266 Free PMC article.
-
Chimeric antigen receptor containing ICOS signaling domain mediates specific and efficient antitumor effect of T cells against EGFRvIII expressing glioma.J Hematol Oncol. 2013 May 9;6:33. doi: 10.1186/1756-8722-6-33. J Hematol Oncol. 2013. PMID: 23656794 Free PMC article.
-
Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma.Hum Gene Ther. 2012 Oct;23(10):1043-53. doi: 10.1089/hum.2012.041. Epub 2012 Sep 24. Hum Gene Ther. 2012. PMID: 22780919 Free PMC article.
-
Tumor-specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma.Semin Immunol. 2008 Oct;20(5):267-75. doi: 10.1016/j.smim.2008.04.001. Epub 2008 Jun 9. Semin Immunol. 2008. PMID: 18539480 Free PMC article. Review.
-
Anti-EGFRvIII Chimeric Antigen Receptor-Modified T Cells for Adoptive Cell Therapy of Glioblastoma.Curr Pharm Des. 2017;23(14):2113-2116. doi: 10.2174/1381612823666170316125402. Curr Pharm Des. 2017. PMID: 28302023 Free PMC article. Review.
Cited by
-
Intravital imaging reveals synergistic effect of CAR T-cells and radiation therapy in a preclinical immunocompetent glioblastoma model.Oncoimmunology. 2020 May 13;9(1):1757360. doi: 10.1080/2162402X.2020.1757360. Oncoimmunology. 2020. PMID: 32923113 Free PMC article.
-
Immune Escape After Adoptive T-cell Therapy for Malignant Gliomas.Clin Cancer Res. 2020 Nov 1;26(21):5689-5700. doi: 10.1158/1078-0432.CCR-20-1065. Epub 2020 Aug 11. Clin Cancer Res. 2020. PMID: 32788225 Free PMC article.
-
Chimeric antigen receptor-modified T cells for the treatment of solid tumors: Defining the challenges and next steps.Pharmacol Ther. 2016 Oct;166:30-9. doi: 10.1016/j.pharmthera.2016.06.010. Epub 2016 Jun 29. Pharmacol Ther. 2016. PMID: 27373504 Free PMC article. Review.
-
Targeting and killing glioblastoma with monoclonal antibody to O-acetyl GD2 ganglioside.Oncotarget. 2016 Jul 5;7(27):41172-41185. doi: 10.18632/oncotarget.9226. Oncotarget. 2016. PMID: 27172791 Free PMC article.
-
Long-term in vivo microscopy of CAR T cell dynamics during eradication of CNS lymphoma in mice.Proc Natl Acad Sci U S A. 2019 Nov 26;116(48):24275-24284. doi: 10.1073/pnas.1903854116. Epub 2019 Nov 11. Proc Natl Acad Sci U S A. 2019. PMID: 31712432 Free PMC article.
References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. - PubMed
-
- Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:917–924. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

