γ-Secretase processing and effects of γ-secretase inhibitors and modulators on long Aβ peptides in cells
- PMID: 24352661
- PMCID: PMC3916530
- DOI: 10.1074/jbc.M113.512921
γ-Secretase processing and effects of γ-secretase inhibitors and modulators on long Aβ peptides in cells
Abstract
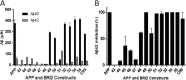
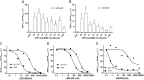
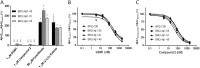
Understanding how different species of Aβ are generated by γ-secretase cleavage has broad therapeutic implications, because shifts in γ-secretase processing that increase the relative production of Aβx-42/43 can initiate a pathological cascade, resulting in Alzheimer disease. We have explored the sequential stepwise γ-secretase cleavage model in cells. Eighteen BRI2-Aβ fusion protein expression constructs designed to generate peptides from Aβ1-38 to Aβ1-55 and C99 (CTFβ) were transfected into cells, and Aβ production was assessed. Secreted and cell-associated Aβ were detected using ELISA and immunoprecipitation MALDI-TOF mass spectrometry. Aβ peptides from 1-38 to 1-55 were readily detected in the cells and as soluble full-length Aβ proteins in the media. Aβ peptides longer than Aβ1-48 were efficiently cleaved by γ-secretase and produced varying ratios of Aβ1-40:Aβ1-42. γ-Secretase cleavage of Aβ1-51 resulted in much higher levels of Aβ1-42 than any other long Aβ peptides, but the processing of Aβ1-51 was heterogeneous with significant amounts of shorter Aβs, including Aβ1-40, produced. Two PSEN1 variants altered Aβ1-42 production from Aβ1-51 but not Aβ1-49. Unexpectedly, long Aβ peptide substrates such as Aβ1-49 showed reduced sensitivity to inhibition by γ-secretase inhibitors. In contrast, long Aβ substrates showed little differential sensitivity to multiple γ-secretase modulators. Although these studies further support the sequential γ-secretase cleavage model, they confirm that in cells the initial γ-secretase cleavage does not precisely define subsequent product lines. These studies also raise interesting issues about the solubility and detection of long Aβ, as well as the use of truncated substrates for assessing relative potency of γ-secretase inhibitors.
Keywords: Alzheimer Disease; Amyloid; Amyloid Precursor Protein; Intramembrane Proteolysis; Protease.
Figures


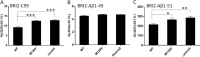
Similar articles
-
Effects of gamma-secretase inhibition on the amyloid beta isoform pattern in a mouse model of Alzheimer's disease.Neurodegener Dis. 2009;6(5-6):258-62. doi: 10.1159/000264639. Epub 2009 Dec 3. Neurodegener Dis. 2009. PMID: 19955704 Free PMC article.
-
Alzheimer presenilin-1 mutations dramatically reduce trimming of long amyloid β-peptides (Aβ) by γ-secretase to increase 42-to-40-residue Aβ.J Biol Chem. 2014 Nov 7;289(45):31043-52. doi: 10.1074/jbc.M114.581165. Epub 2014 Sep 19. J Biol Chem. 2014. PMID: 25239621 Free PMC article.
-
Independent generation of Abeta42 and Abeta38 peptide species by gamma-secretase.J Biol Chem. 2008 Jun 20;283(25):17049-54. doi: 10.1074/jbc.M802912200. Epub 2008 Apr 21. J Biol Chem. 2008. PMID: 18426795
-
gamma-Secretase as a therapeutic target in Alzheimer's disease.Curr Drug Targets. 2010 Apr;11(4):506-17. doi: 10.2174/138945010790980349. Curr Drug Targets. 2010. PMID: 20015011 Review.
-
Dynamic Nature of presenilin1/γ-Secretase: Implication for Alzheimer's Disease Pathogenesis.Mol Neurobiol. 2018 Mar;55(3):2275-2284. doi: 10.1007/s12035-017-0487-5. Epub 2017 Mar 22. Mol Neurobiol. 2018. PMID: 28332150 Free PMC article. Review.
Cited by
-
Differential Inhibition of Signal Peptide Peptidase Family Members by Established γ-Secretase Inhibitors.PLoS One. 2015 Jun 5;10(6):e0128619. doi: 10.1371/journal.pone.0128619. eCollection 2015. PLoS One. 2015. PMID: 26046535 Free PMC article.
-
Individual and combined presenilin 1 and 2 knockouts reveal that both have highly overlapping functions in HEK293T cells.J Biol Chem. 2019 Jul 19;294(29):11276-11285. doi: 10.1074/jbc.RA119.008041. Epub 2019 Jun 5. J Biol Chem. 2019. PMID: 31167792 Free PMC article.
-
γ-Secretase inhibitors in cancer clinical trials are pharmacologically and functionally distinct.EMBO Mol Med. 2017 Jul;9(7):950-966. doi: 10.15252/emmm.201607265. EMBO Mol Med. 2017. PMID: 28539479 Free PMC article.
-
Interrelationship between Changes in the Amyloid β 42/40 Ratio and Presenilin 1 Conformation.Mol Med. 2016 Sep;22:329-337. doi: 10.2119/molmed.2016.00127. Epub 2016 Jul 5. Mol Med. 2016. PMID: 27391800 Free PMC article.
-
Modulation of Aβ42 in vivo by γ-secretase modulator in primates and humans.Alzheimers Res Ther. 2015 Aug 5;7(1):55. doi: 10.1186/s13195-015-0137-y. eCollection 2015. Alzheimers Res Ther. 2015. PMID: 26244059 Free PMC article.
References
-
- Hardy J., Selkoe D. J. (2002) The amyloid hypothesis of Alzheimer's disease. Progress and problems on the road to therapeutics. Science 297, 353–356 - PubMed
-
- Zou K., Liu J., Watanabe A., Hiraga S., Liu S., Tanabe C., Maeda T., Terayama Y., Takahashi S., Michikawa M., Komano H. (2013) Aβ43 is the earliest-depositing Aβ species in APP transgenic mouse brain and is converted to Aβ41 by two active domains of ACE. Am. J. Pathol. 182, 2322–2331 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

