Evidence for hysteretic substrate channeling in the proline dehydrogenase and Δ1-pyrroline-5-carboxylate dehydrogenase coupled reaction of proline utilization A (PutA)
- PMID: 24352662
- PMCID: PMC3916563
- DOI: 10.1074/jbc.M113.523704
Evidence for hysteretic substrate channeling in the proline dehydrogenase and Δ1-pyrroline-5-carboxylate dehydrogenase coupled reaction of proline utilization A (PutA)
Abstract
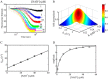
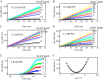
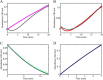
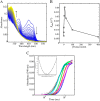
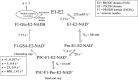
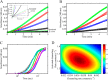
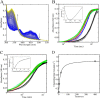
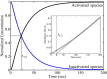
PutA (proline utilization A) is a large bifunctional flavoenzyme with proline dehydrogenase (PRODH) and Δ(1)-pyrroline-5-carboxylate dehydrogenase (P5CDH) domains that catalyze the oxidation of l-proline to l-glutamate in two successive reactions. In the PRODH active site, proline undergoes a two-electron oxidation to Δ(1)-pyrroline-5-carboxlylate, and the FAD cofactor is reduced. In the P5CDH active site, l-glutamate-γ-semialdehyde (the hydrolyzed form of Δ(1)-pyrroline-5-carboxylate) undergoes a two-electron oxidation in which a hydride is transferred to NAD(+)-producing NADH and glutamate. Here we report the first kinetic model for the overall PRODH-P5CDH reaction of a PutA enzyme. Global analysis of steady-state and transient kinetic data for the PRODH, P5CDH, and coupled PRODH-P5CDH reactions was used to test various models describing the conversion of proline to glutamate by Escherichia coli PutA. The coupled PRODH-P5CDH activity of PutA is best described by a mechanism in which the intermediate is not released into the bulk medium, i.e., substrate channeling. Unexpectedly, single-turnover kinetic experiments of the coupled PRODH-P5CDH reaction revealed that the rate of NADH formation is 20-fold slower than the steady-state turnover number for the overall reaction, implying that catalytic cycling speeds up throughput. We show that the limiting rate constant observed for NADH formation in the first turnover increases by almost 40-fold after multiple turnovers, achieving half of the steady-state value after 15 turnovers. These results suggest that EcPutA achieves an activated channeling state during the approach to steady state and is thus a new example of a hysteretic enzyme. Potential underlying causes of activation of channeling are discussed.
Keywords: Enzyme Kinetics; Enzyme Mechanisms; Enzyme Turnover; Flavin; Hysteresis; Metabolism; Proline; Substrate Channeling.
Figures
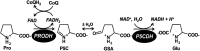

Similar articles
-
First evidence for substrate channeling between proline catabolic enzymes: a validation of domain fusion analysis for predicting protein-protein interactions.J Biol Chem. 2015 Jan 23;290(4):2225-34. doi: 10.1074/jbc.M114.625483. Epub 2014 Dec 9. J Biol Chem. 2015. PMID: 25492892 Free PMC article.
-
Identification of a Conserved Histidine As Being Critical for the Catalytic Mechanism and Functional Switching of the Multifunctional Proline Utilization A Protein.Biochemistry. 2017 Jun 20;56(24):3078-3088. doi: 10.1021/acs.biochem.7b00046. Epub 2017 Jun 8. Biochemistry. 2017. PMID: 28558236 Free PMC article.
-
Exploring the proline-dependent conformational change in the multifunctional PutA flavoprotein by tryptophan fluorescence spectroscopy.Biochemistry. 2005 Sep 20;44(37):12297-306. doi: 10.1021/bi051026b. Biochemistry. 2005. PMID: 16156643
-
Structure, function, and mechanism of proline utilization A (PutA).Arch Biochem Biophys. 2017 Oct 15;632:142-157. doi: 10.1016/j.abb.2017.07.005. Epub 2017 Jul 14. Arch Biochem Biophys. 2017. PMID: 28712849 Free PMC article. Review.
-
Substrate channeling in proline metabolism.Front Biosci (Landmark Ed). 2012 Jan 1;17(1):375-88. doi: 10.2741/3932. Front Biosci (Landmark Ed). 2012. PMID: 22201749 Free PMC article. Review.
Cited by
-
Cadmium and Secondary Structure-dependent Function of a Degron in the Pca1p Cadmium Exporter.J Biol Chem. 2016 Jun 3;291(23):12420-31. doi: 10.1074/jbc.M116.724930. Epub 2016 Apr 8. J Biol Chem. 2016. PMID: 27059957 Free PMC article.
-
Resolving the cofactor-binding site in the proline biosynthetic enzyme human pyrroline-5-carboxylate reductase 1.J Biol Chem. 2017 Apr 28;292(17):7233-7243. doi: 10.1074/jbc.M117.780288. Epub 2017 Mar 3. J Biol Chem. 2017. PMID: 28258219 Free PMC article.
-
Role of Proline in Pathogen and Host Interactions.Antioxid Redox Signal. 2019 Feb 1;30(4):683-709. doi: 10.1089/ars.2017.7335. Epub 2018 Feb 2. Antioxid Redox Signal. 2019. PMID: 29241353 Free PMC article. Review.
-
Discovery of the Membrane Binding Domain in Trifunctional Proline Utilization A.Biochemistry. 2017 Nov 28;56(47):6292-6303. doi: 10.1021/acs.biochem.7b01008. Epub 2017 Nov 15. Biochemistry. 2017. PMID: 29090935 Free PMC article.
-
Structure and characterization of a class 3B proline utilization A: Ligand-induced dimerization and importance of the C-terminal domain for catalysis.J Biol Chem. 2017 Jun 9;292(23):9652-9665. doi: 10.1074/jbc.M117.786855. Epub 2017 Apr 18. J Biol Chem. 2017. PMID: 28420730 Free PMC article.
References
-
- Szabados L., Savouré A. (2010) Proline. A multifunctional amino acid. Trends Plant Sci. 15, 89–97 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

