Modelling the mechanics of partially mineralized collagen fibrils, fibres and tissue
- PMID: 24352669
- PMCID: PMC3899858
- DOI: 10.1098/rsif.2013.0835
Modelling the mechanics of partially mineralized collagen fibrils, fibres and tissue
Abstract
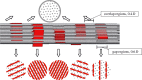
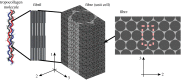
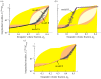
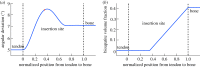
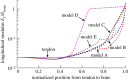
Progressive stiffening of collagen tissue by bioapatite mineral is important physiologically, but the details of this stiffening are uncertain. Unresolved questions about the details of the accommodation of bioapatite within and upon collagen's hierarchical structure have posed a central hurdle, but recent microscopy data resolve several major questions. These data suggest how collagen accommodates bioapatite at the lowest relevant hierarchical level (collagen fibrils), and suggest several possibilities for the progressive accommodation of bioapatite at higher hierarchical length scales (fibres and tissue). We developed approximations for the stiffening of collagen across spatial hierarchies based upon these data, and connected models across hierarchies levels to estimate mineralization-dependent tissue-level mechanics. In the five possible sequences of mineralization studied, percolation of the bioapatite phase proved to be an important determinant of the degree of stiffening by bioapatite. The models were applied to study one important instance of partially mineralized tissue, which occurs at the attachment of tendon to bone. All sequences of mineralization considered reproduced experimental observations of a region of tissue between tendon and bone that is more compliant than either tendon or bone, but the size and nature of this region depended strongly upon the sequence of mineralization. These models and observations have implications for engineered tissue scaffolds at the attachment of tendon to bone, bone development and graded biomimetic attachment of dissimilar hierarchical materials in general.
Keywords: bone; mechanics of developing tissues; mineralization; mineralized fibrils; nanomechanics; tendon-to-bone attachment.
Figures



Similar articles
-
Functional grading of mineral and collagen in the attachment of tendon to bone.Biophys J. 2009 Aug 19;97(4):976-85. doi: 10.1016/j.bpj.2009.05.043. Biophys J. 2009. PMID: 19686644 Free PMC article.
-
The nanometre-scale physiology of bone: steric modelling and scanning transmission electron microscopy of collagen-mineral structure.J R Soc Interface. 2012 Aug 7;9(73):1774-86. doi: 10.1098/rsif.2011.0880. Epub 2012 Feb 16. J R Soc Interface. 2012. PMID: 22345156 Free PMC article.
-
Synchrotron diffraction study of deformation mechanisms in mineralized tendon.Phys Rev Lett. 2004 Oct 8;93(15):158101. doi: 10.1103/PhysRevLett.93.158101. Epub 2004 Oct 4. Phys Rev Lett. 2004. PMID: 15524943
-
Structure-mechanics relationships in mineralized tendons.J Mech Behav Biomed Mater. 2015 Dec;52:72-84. doi: 10.1016/j.jmbbm.2015.03.013. Epub 2015 Apr 1. J Mech Behav Biomed Mater. 2015. PMID: 25922092 Review.
-
An overview of vertebrate mineralization with emphasis on collagen-mineral interaction.Gravit Space Biol Bull. 1999 May;12(2):15-26. Gravit Space Biol Bull. 1999. PMID: 11541779 Review.
Cited by
-
Effect of chitosan and CMCS on dentin after Er:YAG laser irradiation: shear bond strength and surface morphology analysis.BMC Oral Health. 2024 Mar 29;24(1):402. doi: 10.1186/s12903-024-04097-w. BMC Oral Health. 2024. PMID: 38553692 Free PMC article.
-
Spatiotemporally Controlled Photoresponsive Hydrogels: Design and Predictive Modeling from Processing through Application.Adv Funct Mater. 2020 Aug 7;30(32):2000639. doi: 10.1002/adfm.202000639. Epub 2020 Jun 18. Adv Funct Mater. 2020. PMID: 32802013 Free PMC article. Review.
-
Adhesive-based tendon-to-bone repair: failure modelling and materials selection.J R Soc Interface. 2019 Apr 26;16(153):20180838. doi: 10.1098/rsif.2018.0838. J R Soc Interface. 2019. PMID: 30966948 Free PMC article.
-
Regularization-Free Strain Mapping in Three Dimensions, With Application to Cardiac Ultrasound.J Biomech Eng. 2019 Jan 1;141(1):0110101-01101011. doi: 10.1115/1.4041576. J Biomech Eng. 2019. PMID: 30267039 Free PMC article.
-
Acoustic radiation force on a long cylinder, and potential sound transduction by tomato trichomes.Biophys J. 2022 Oct 18;121(20):3917-3926. doi: 10.1016/j.bpj.2022.08.038. Epub 2022 Aug 30. Biophys J. 2022. PMID: 36045574 Free PMC article.
References
-
- Glimcher MJ. 1987. The nature of the mineral component of bone and the mechanism of calcification. Instr. Course Lect. 36, 49–69. - PubMed
-
- Alexander B, Daulton TL, Genin GM, Lipner J, Pasteris JD, Wopenka B, Thomopoulos S. 2012. The nanometre-scale physiology of bone: steric modelling and scanning transmission electron microscopy of collagen–mineral structure. J. R. Soc. Interface 9, 1774–1786. (10.1098/rsif.2011.0880) - DOI - PMC - PubMed
-
- Ji B, Gao H. 2006. Elastic properties of nanocomposite structure of bone. Compos. Sci. Technol. 66, 1212–1218. (10.1016/j.compscitech.2005.10.017) - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

