Microbial alkyl- and aryl-sulfatases: mechanism, occurrence, screening and stereoselectivities
- PMID: 24352732
- PMCID: PMC3920027
- DOI: 10.1007/s00253-013-5438-0
Microbial alkyl- and aryl-sulfatases: mechanism, occurrence, screening and stereoselectivities
Abstract
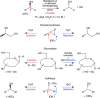
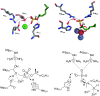
This review gives an overview on the occurrence of sulfatases in Prokaryota, Eukaryota and Archaea. The mechanism of enzymes acting with retention or inversion of configuration during sulfate ester hydrolysis is discussed taking two complementary examples. Methods for the discovery of novel alkyl sulfatases are described by way of sequence-based search and enzyme induction. A comprehensive list of organisms with their respective substrate scope regarding prim- and sec-alkyl sulfate esters allows to assess the capabilities and limitations of various biocatalysts employed as whole cell systems or as purified enzymes with respect to their activities and enantioselectivities. Methods for immobilization and selectivity enhancement by addition of metal ions or organic (co)solvents are summarised.
Figures

Similar articles
-
Purification and characterization of an inverting stereo- and enantioselective sec-alkylsulfatase from the gram-positive bacterium Rhodococcus ruber DSM 44541.Appl Environ Microbiol. 2003 May;69(5):2810-5. doi: 10.1128/AEM.69.5.2810-2815.2003. Appl Environ Microbiol. 2003. PMID: 12732552 Free PMC article.
-
New enzymes for biotransformations: microbial alkyl sulfatases displaying stereo- and enantioselectivity.Trends Biotechnol. 2007 Feb;25(2):83-8. doi: 10.1016/j.tibtech.2006.11.006. Epub 2006 Dec 5. Trends Biotechnol. 2007. PMID: 17150269 Review.
-
Highly enantioselective stereo-inverting sec-alkylsulfatase activity of hyperthermophilic Archaea.Org Biomol Chem. 2005 Jul 21;3(14):2652-6. doi: 10.1039/b504883d. Epub 2005 Jun 21. Org Biomol Chem. 2005. PMID: 15999201
-
Highly enantioselective sec-alkyl sulfatase activity of Sulfolobus acidocaldarius DSM 639.Org Lett. 2004 Dec 23;6(26):5009-10. doi: 10.1021/ol0477778. Org Lett. 2004. PMID: 15606122
-
New enzymes for biotransformations.Curr Opin Chem Biol. 2005 Apr;9(2):181-7. doi: 10.1016/j.cbpa.2005.01.001. Curr Opin Chem Biol. 2005. PMID: 15811803 Review.
Cited by
-
Expanding the β-Lactamase Family in the Human Microbiome.Adv Sci (Weinh). 2024 Dec;11(46):e2403563. doi: 10.1002/advs.202403563. Epub 2024 Oct 24. Adv Sci (Weinh). 2024. PMID: 39447121 Free PMC article.
-
Tyrosine sulfation as a protein post-translational modification.Molecules. 2015 Jan 28;20(2):2138-64. doi: 10.3390/molecules20022138. Molecules. 2015. PMID: 25635379 Free PMC article. Review.
-
Sulfur Cycling and the Intestinal Microbiome.Dig Dis Sci. 2017 Sep;62(9):2241-2257. doi: 10.1007/s10620-017-4689-5. Epub 2017 Aug 1. Dig Dis Sci. 2017. PMID: 28766244 Review.
-
MaAts, an Alkylsulfatase, Contributes to Fungal Tolerances against UV-B Irradiation and Heat-Shock in Metarhizium acridum.J Fungi (Basel). 2022 Mar 8;8(3):270. doi: 10.3390/jof8030270. J Fungi (Basel). 2022. PMID: 35330272 Free PMC article.
-
Unique Features of Entamoeba Sulfur Metabolism; Compartmentalization, Physiological Roles of Terminal Products, Evolution and Pharmaceutical Exploitation.Int J Mol Sci. 2019 Sep 21;20(19):4679. doi: 10.3390/ijms20194679. Int J Mol Sci. 2019. PMID: 31546588 Free PMC article. Review.
References
-
- Abboud MM, Khleifat KM, Batarseh M, Tarawneh KA, Al-Mustafa A, Al-Madadhah M. Different optimization conditions required for enhancing the biodegradation of linear alkylbenzosulfonate and sodium dodecyl sulfate surfactants by novel consortium of Acinetobacter calcoaceticus and Pantoea agglomerans. Enzyme Microb Technol. 2007;41:432–439. doi: 10.1016/j.enzmictec.2007.03.011. - DOI
-
- Ambily PS, Jisha MS. Biodegradation of anionic surfactant, sodium dodecyl sulphate by Pseudomonas aeruginosa MTCC 10311. J Environ Biol. 2012;33:717–720. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

