Neuron-glia crosstalk in health and disease: fractalkine and CX3CR1 take centre stage
- PMID: 24352739
- PMCID: PMC3877844
- DOI: 10.1098/rsob.130181
Neuron-glia crosstalk in health and disease: fractalkine and CX3CR1 take centre stage
Abstract
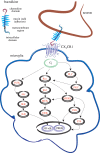
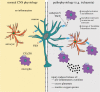
An essential aspect of normal brain function is the bidirectional interaction and communication between neurons and neighbouring glial cells. To this end, the brain has evolved ligand-receptor partnerships that facilitate crosstalk between different cell types. The chemokine, fractalkine (FKN), is expressed on neuronal cells, and its receptor, CX(3)CR1, is predominantly expressed on microglia. This review focuses on several important functional roles for FKN/CX(3)CR1 in both health and disease of the central nervous system. It has been posited that FKN is involved in microglial infiltration of the brain during development. Microglia, in turn, are implicated in the developmental synaptic pruning that occurs during brain maturation. The abundance of FKN on mature hippocampal neurons suggests a homeostatic non-inflammatory role in mechanisms of learning and memory. There is substantial evidence describing a role for FKN in hippocampal synaptic plasticity. FKN, on the one hand, appears to prevent excess microglial activation in the absence of injury while promoting activation of microglia and astrocytes during inflammatory episodes. Thus, FKN appears to be neuroprotective in some settings, whereas it contributes to neuronal damage in others. Many progressive neuroinflammatory disorders that are associated with increased microglial activation, such as Alzheimer's disease, show disruption of the FKN/CX(3)CR1 communication system. Thus, targeting CX(3)CR1 receptor hyperactivation with specific antagonists in such neuroinflammatory conditions may eventually lead to novel neurotherapeutics.
Keywords: Alzheimer's disease; CX3CR1; fractalkine; ischaemia; microglia; synaptic plasticity.
Figures

References
-
- Kriegstein AR, Götz M. 2003. Radial glia diversity: a matter of cell fate. Glia 43, 37–43 (doi:10.1002/glia.10250) - DOI - PubMed
-
- Paukert M, Bergles DE. 2006. Synaptic communication between neurons and NG2+ cells. Curr. Opin. Neurobiol. 16, 515–521 (doi:10.1016/j.conb.2006.08.009) - DOI - PubMed
-
- Nishiyama A. 2007. Polydendrocytes: NG2 cells with many roles in development and repair of the CNS. Neuroscientist 13, 62–76 (doi:10.1177/1073858406295586) - DOI - PubMed
-
- Pinto L, Götz M. 2007. Radial glial cell heterogeneity: the source of diverse progeny in the CNS. Prog. Neurobiol. 83, 2–23 (doi:10.1016/j.pneurobio.2007.02.010) - DOI - PubMed
-
- Kettenmann H, Verkhratsky A. 2008. Neuroglia: the 150 years after. Trends Neurosci. 31, 653–659 (doi:10.1016/j.tins.2008.09.003) - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
