Efficacy of erlotinib in patients with relapsed gliobastoma multiforme who expressed EGFRVIII and PTEN determined by immunohistochemistry
- PMID: 24352766
- PMCID: PMC3890043
- DOI: 10.1007/s11060-013-1316-y
Efficacy of erlotinib in patients with relapsed gliobastoma multiforme who expressed EGFRVIII and PTEN determined by immunohistochemistry
Erratum in
- J Neurooncol. 2014 Dec;120(3):667
Abstract
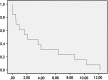
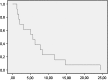
Epidermal growth factor receptor gene (EGFR) alteration is a common feature in most of glioblastoma multiforme (GBM). Robust response of anti-EGFR treatments has been mostly associated with the EGFR deletion mutant variant III (EGFRvIII) and expression of PTEN. We have performed a prospective trial in order to confirm the efficacy of erlotinib treatment in patients with relapsed GBM who expressed EGFRvIII and PTEN. All patients included in the trial were required to be PTEN (+++), EGFR (+++) and EGFRvIII (+++) positives by immunohistochemistry. This new phase II trial enrolled 40 patients and was design to be stopped in case of fewer than two responses in the first 13 patients. Patient eligibility included histopathology criteria, radiological progression, more than 18 years old, Karnofsky performed status, KPS > 50, and adequate bone marrow and organ function. There was no limit to the number of prior treatments for relapses. No enzyme-inducing antiepileptic drugs were allowed. The primary endpoints were response and progression-free survival at 6 months (PFS6). Thirteen patients (6 men, 7 women) with recurrent GBM received erlotinib 150 mg/day. Median age was 53 years, median KPS was 80, and median prior treatments for relapses were 2. There was one partial response and three stable diseases (one at 18 months). PFS at 6 months was 20 %. Dose reduction for toxicity was not needed in any patient. Dermatitis was the main treatment-related toxicity, grade 1 in 8 patients and grade 2 in 5 patients. No grade 3 toxicity was observed. Median survival was 7 months (95 % IC 1.41-4.7). As conclusion, monotherapy with erlotinib in GBM relapses patients with high protein expression for PTEN (+++), EGFR (+++), and EGFRvlII (+++) showed low toxicity but minimal efficacy and the trial stopped.
Figures
Similar articles
-
Targeted therapy with bevacizumab and erlotinib tailored to the molecular profile of patients with recurrent glioblastoma. Preliminary experience.Acta Neurochir (Wien). 2013 Jan;155(1):33-40. doi: 10.1007/s00701-012-1536-5. Epub 2012 Nov 8. Acta Neurochir (Wien). 2013. PMID: 23132371 Clinical Trial.
-
Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034.J Clin Oncol. 2009 Mar 10;27(8):1268-74. doi: 10.1200/JCO.2008.17.5984. Epub 2009 Feb 9. J Clin Oncol. 2009. PMID: 19204207 Free PMC article. Clinical Trial.
-
Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma.J Clin Oncol. 2009 Feb 1;27(4):579-84. doi: 10.1200/JCO.2008.18.9639. Epub 2008 Dec 15. J Clin Oncol. 2009. PMID: 19075262 Free PMC article. Clinical Trial.
-
The EGFRvIII variant in glioblastoma multiforme.J Clin Neurosci. 2009 Jun;16(6):748-54. doi: 10.1016/j.jocn.2008.12.005. Epub 2009 Mar 25. J Clin Neurosci. 2009. PMID: 19324552 Review.
-
Clinical outcomes of gamma knife radiosurgery in the salvage treatment of patients with recurrent high-grade glioma.World Neurosurg. 2013 Dec;80(6):872-8. doi: 10.1016/j.wneu.2013.02.030. Epub 2013 Feb 9. World Neurosurg. 2013. PMID: 23403349 Review.
Cited by
-
Congress of Neurological Surgeons systematic review and evidence-based guidelines update on the role of targeted therapies and immunotherapies in the management of progressive glioblastoma.J Neurooncol. 2022 Jun;158(2):265-321. doi: 10.1007/s11060-021-03876-7. Epub 2021 Oct 25. J Neurooncol. 2022. PMID: 34694567 Free PMC article.
-
Network Analyses of Brain Tumor Patients' Multiomic Data Reveals Pharmacological Opportunities to Alter Cell State Transitions.bioRxiv [Preprint]. 2024 May 10:2024.05.08.593202. doi: 10.1101/2024.05.08.593202. bioRxiv. 2024. Update in: NPJ Syst Biol Appl. 2025 Feb 01;11(1):14. doi: 10.1038/s41540-025-00493-2. PMID: 38766170 Free PMC article. Updated. Preprint.
-
Toward precision medicine in glioblastoma: the promise and the challenges.Neuro Oncol. 2015 Aug;17(8):1051-63. doi: 10.1093/neuonc/nov031. Epub 2015 May 1. Neuro Oncol. 2015. PMID: 25934816 Free PMC article. Review.
-
Exploring Predictors of Response to Dacomitinib in EGFR-Amplified Recurrent Glioblastoma.JCO Precis Oncol. 2020 Jun 8;4:PO.19.00295. doi: 10.1200/PO.19.00295. eCollection 2020. JCO Precis Oncol. 2020. PMID: 32923886 Free PMC article.
-
Mechanistic insights and the clinical prospects of targeted therapies for glioblastoma: a comprehensive review.Exp Hematol Oncol. 2024 Apr 13;13(1):40. doi: 10.1186/s40164-024-00512-8. Exp Hematol Oncol. 2024. PMID: 38615034 Free PMC article. Review.
References
-
- Van den Bent MJ, Brandes A, Rampling R, Kouwenhoven MC, et al. Randomized phase II trial of erlotinib (E) vs. temozolomide (TMZ) or BCNU in recurrent glioblastoma multiforme (GBM): EORTC Brain Tumor Group Study 26034. J Clin Oncol. 2009;10(27):1268–1274. doi: 10.1200/JCO.2008.17.5984. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous