Homologous and heterologous desensitization of guanylyl cyclase-B signaling in GH3 somatolactotropes
- PMID: 24352806
- PMCID: PMC3921447
- DOI: 10.1007/s00441-013-1763-y
Homologous and heterologous desensitization of guanylyl cyclase-B signaling in GH3 somatolactotropes
Abstract
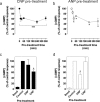
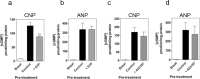
The guanylyl cyclases, GC-A and GC-B, are selective receptors for atrial and C-type natriuretic peptides (ANP and CNP, respectively). In the anterior pituitary, CNP and GC-B are major regulators of cGMP production in gonadotropes and yet mouse models of disrupted CNP and GC-B indicate a potential role in growth hormone secretion. In the current study, we investigate the molecular and pharmacological properties of the CNP/GC-B system in somatotrope lineage cells. Primary rat pituitary and GH3 somatolactotropes expressed functional GC-A and GC-B receptors that had similar EC50 properties in terms of cGMP production. Interestingly, GC-B signaling underwent rapid homologous desensitization in a protein phosphatase 2A (PP2A)-dependent manner. Chronic exposure to either CNP or ANP caused a significant down-regulation of both GC-A- and GC-B-dependent cGMP accumulation in a ligand-specific manner. However, this down-regulation was not accompanied by alterations in the sub-cellular localization of these receptors. Heterologous desensitization of GC-B signaling occurred in GH3 cells following exposure to either sphingosine-1-phosphate or thyrotrophin-releasing hormone (TRH). This heterologous desensitization was protein kinase C (PKC)-dependent, as pre-treatment with GF109203X prevented the effect of TRH on CNP/GC-B signaling. Collectively, these data indicate common and distinct properties of particulate guanylyl cyclase receptors in somatotropes and reveal that independent mechanisms of homologous and heterologous desensitization occur involving either PP2A or PKC. Guanylyl cyclase receptors thus represent potential novel therapeutic targets for treating growth-hormone-associated disorders.
Figures





Similar articles
-
Natriuretic peptide activation of extracellular regulated kinase 1/2 (ERK1/2) pathway by particulate guanylyl cyclases in GH3 somatolactotropes.Cell Tissue Res. 2017 Sep;369(3):567-578. doi: 10.1007/s00441-017-2624-x. Epub 2017 Apr 27. Cell Tissue Res. 2017. PMID: 28451751 Free PMC article.
-
Natriuretic Peptide Expression and Function in GH3 Somatolactotropes and Feline Somatotrope Pituitary Tumours.Int J Mol Sci. 2021 Jan 22;22(3):1076. doi: 10.3390/ijms22031076. Int J Mol Sci. 2021. PMID: 33499110 Free PMC article.
-
C-type natriuretic peptide (CNP) effects in anterior pituitary cell lines: evidence for homologous desensitisation of CNP-stimulated cGMP accumulation in alpha T3-1 gonadotroph-derived cells.J Endocrinol. 2000 Jul;166(1):195-203. doi: 10.1677/joe.0.1660195. J Endocrinol. 2000. PMID: 10856898
-
Regulation and therapeutic targeting of peptide-activated receptor guanylyl cyclases.Pharmacol Ther. 2011 Apr;130(1):71-82. doi: 10.1016/j.pharmthera.2010.12.005. Epub 2010 Dec 24. Pharmacol Ther. 2011. PMID: 21185863 Free PMC article. Review.
-
C-type natriuretic peptide and guanylyl cyclase B receptor.Peptides. 2005 Jun;26(6):1024-34. doi: 10.1016/j.peptides.2004.08.027. Epub 2005 Apr 15. Peptides. 2005. PMID: 15911070 Review.
Cited by
-
Natriuretic peptide activation of extracellular regulated kinase 1/2 (ERK1/2) pathway by particulate guanylyl cyclases in GH3 somatolactotropes.Cell Tissue Res. 2017 Sep;369(3):567-578. doi: 10.1007/s00441-017-2624-x. Epub 2017 Apr 27. Cell Tissue Res. 2017. PMID: 28451751 Free PMC article.
-
Pharmacological and Genetic Disruption of C-Type Natriuretic Peptide (nppcl) Expression in Zebrafish (Danio rerio) Causes Stunted Growth during Development.Int J Mol Sci. 2023 Aug 18;24(16):12921. doi: 10.3390/ijms241612921. Int J Mol Sci. 2023. PMID: 37629102 Free PMC article.
-
Natriuretic Peptide Expression and Function in GH3 Somatolactotropes and Feline Somatotrope Pituitary Tumours.Int J Mol Sci. 2021 Jan 22;22(3):1076. doi: 10.3390/ijms22031076. Int J Mol Sci. 2021. PMID: 33499110 Free PMC article.
-
Sensitivity of the Natriuretic Peptide/cGMP System to Hyperammonaemia in Rat C6 Glioma Cells and GPNT Brain Endothelial Cells.Cells. 2021 Feb 15;10(2):398. doi: 10.3390/cells10020398. Cells. 2021. PMID: 33672024 Free PMC article.
-
Dephosphorylation and inactivation of NPR2 guanylyl cyclase in granulosa cells contributes to the LH-induced decrease in cGMP that causes resumption of meiosis in rat oocytes.Development. 2014 Sep;141(18):3594-604. doi: 10.1242/dev.112219. Development. 2014. PMID: 25183874 Free PMC article.
References
-
- Abou-Samra AB, Catt KJ, Aguilera G. Synthetic atrial natriuretic factors (ANFs) stimulate guanosine 3’,5’-monophosphate production but not hormone release in rat pituitary cells: peptide contamination with a gonadotropin-releasing hormone agonist explains luteinizing hormone-releasing activity of certain ANFs. Endocrinology. 1987;120:18–24. doi: 10.1210/endo-120-1-18. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

