Notch signaling is required for the formation of mesangial cells from a stromal mesenchyme precursor during kidney development
- PMID: 24353058
- PMCID: PMC4074211
- DOI: 10.1242/dev.100271
Notch signaling is required for the formation of mesangial cells from a stromal mesenchyme precursor during kidney development
Abstract
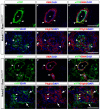
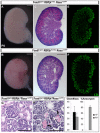
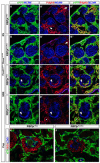
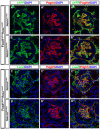
Mesangial cells are specialized pericyte/smooth muscle cells that surround and constrain the vascular network within the glomerulus of the kidney. They are derived from the stromal mesenchyme, a progenitor population distinct from nephron stem cells. Whether mesangial cells have a distinct origin from vascular smooth muscle cells (VSMCs) and the pathways that govern their specification are unknown. Here we show that Notch signaling in stromal progenitors is essential for mesangial cell formation but is dispensable for the smooth muscle and interstitial cell lineages. Deletion of RBPjk, the common DNA-binding partner of all active Notch receptors, with Foxd1(tgCre) results in glomerular aneurysm and perinatal death from kidney failure. This defect occurs early in glomerular development as stromal-derived, desmin-positive cells fail to coalesce near forming nephrons and thus do not invade the vascular cleft of the S-shaped body. This is in contrast to other mutants in which the loss of the mesangium was due to migration defects, and suggests that loss of Notch signaling results in a failure to specify this population from the stroma. Interestingly, Pdgfrb-positive VSMCs do not enter the vascular cleft and cannot rescue the mesangial deficiency. Notch1 and Notch2 act redundantly through γ-secretase and RBPjk in this process, as individual mutants have mesangial cells at birth. Together, these data demonstrate a unique origin of mesangial cells and demonstrate a novel, redundant function for Notch receptors in mesangial cell specification, proliferation or survival during kidney development.
Keywords: Foxd1; Kidney; Mesangium; Mouse; Notch; Progenitors.
Figures




References
-
- Boyle S., Misfeldt A., Chandler K. J., Deal K. K., Southard-Smith E. M., Mortlock D. P., Baldwin H. S., de Caestecker M. (2008). Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev. Biol. 313, 234–245 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

