Postsynaptic glutamate receptors regulate local BMP signaling at the Drosophila neuromuscular junction
- PMID: 24353060
- PMCID: PMC3879819
- DOI: 10.1242/dev.097758
Postsynaptic glutamate receptors regulate local BMP signaling at the Drosophila neuromuscular junction
Abstract
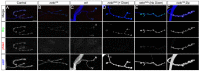
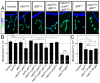
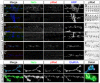
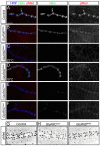
Effective communication between pre- and postsynaptic compartments is required for proper synapse development and function. At the Drosophila neuromuscular junction (NMJ), a retrograde BMP signal functions to promote synapse growth, stability and homeostasis and coordinates the growth of synaptic structures. Retrograde BMP signaling triggers accumulation of the pathway effector pMad in motoneuron nuclei and at synaptic termini. Nuclear pMad, in conjunction with transcription factors, modulates the expression of target genes and instructs synaptic growth; a role for synaptic pMad remains to be determined. Here, we report that pMad signals are selectively lost at NMJ synapses with reduced postsynaptic sensitivities. Despite this loss of synaptic pMad, nuclear pMad persisted in motoneuron nuclei, and expression of BMP target genes was unaffected, indicating a specific impairment in pMad production/maintenance at synaptic termini. During development, synaptic pMad accumulation followed the arrival and clustering of ionotropic glutamate receptors (iGluRs) at NMJ synapses. Synaptic pMad was lost at NMJ synapses developing at suboptimal levels of iGluRs and Neto, an auxiliary subunit required for functional iGluRs. Genetic manipulations of non-essential iGluR subunits revealed that synaptic pMad signals specifically correlated with the postsynaptic type-A glutamate receptors. Altering type-A receptor activities via protein kinase A (PKA) revealed that synaptic pMad depends on the activity and not the net levels of postsynaptic type-A receptors. Thus, synaptic pMad functions as a local sensor for NMJ synapse activity and has the potential to coordinate synaptic activity with a BMP retrograde signal required for synapse growth and homeostasis.
Keywords: BMP signaling; Drosophila; Glutamate receptor; Glutamatergic synapses; Neuromuscular junction.
Figures


References
-
- Aberle H., Haghighi A. P., Fetter R. D., McCabe B. D., Magalhães T. R., Goodman C. S. (2002). wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron 33, 545–558 - PubMed
-
- Adachi-Yamada T., Nakamura M., Irie K., Tomoyasu Y., Sano Y., Mori E., Goto S., Ueno N., Nishida Y., Matsumoto K. (1999). p38 mitogen-activated protein kinase can be involved in transforming growth factor beta superfamily signal transduction in Drosophila wing morphogenesis. Mol. Cell. Biol. 19, 2322–2329 - PMC - PubMed
-
- Ball R. W., Warren-Paquin M., Tsurudome K., Liao E. H., Elazzouzi F., Cavanagh C., An B. S., Wang T. T., White J. H., Haghighi A. P. (2010). Retrograde BMP signaling controls synaptic growth at the NMJ by regulating trio expression in motor neurons. Neuron 66, 536–549 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

