Developing biomarkers in mood disorders research through the use of rapid-acting antidepressants
- PMID: 24353110
- PMCID: PMC3984598
- DOI: 10.1002/da.22224
Developing biomarkers in mood disorders research through the use of rapid-acting antidepressants
Abstract
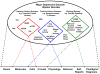
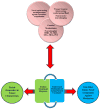
An impediment to progress in mood disorders research is the lack of analytically valid and qualified diagnostic and treatment biomarkers. Consistent with the National Institute of Mental Health (NIMH)'s Research Domain Criteria (RDoC) initiative, the lack of diagnostic biomarkers has precluded us from moving away from a purely subjective (symptom-based) toward a more objective diagnostic system. In addition, treatment response biomarkers in mood disorders would facilitate drug development and move beyond trial-and-error toward more personalized treatments. As such, biomarkers identified early in the pathophysiological process are proximal biomarkers (target engagement), while those occurring later in the disease process are distal (disease pathway components). One strategy to achieve this goal in biomarker development is to increase efforts at the initial phases of biomarker development (i.e. exploration and validation) at single sites with the capability of integrating multimodal approaches across a biological systems level. Subsequently, resultant putative biomarkers could then undergo characterization and surrogacy as these latter phases require multisite collaborative efforts. We have used multimodal approaches - genetics, proteomics/metabolomics, peripheral measures, multimodal neuroimaging, neuropsychopharmacological challenge paradigms and clinical predictors - to explore potential predictor and mediator/moderator biomarkers of the rapid-acting antidepressants ketamine and scopolamine. These exploratory biomarkers may then be used for a priori stratification in larger multisite controlled studies during the validation and characterization phases with the ultimate goal of surrogacy. In sum, the combination of target engagement and well-qualified disease-related measures are crucial to improve our pathophysiological understanding, personalize treatment selection, and expand our armamentarium of novel therapeutics.
Keywords: biomarker; bipolar depression; bipolar disorder; depression; drug development; major depressive disorder; mediator; moderator; neuroimaging; predictor.
© 2013 Wiley Periodicals, Inc.
Figures


References
-
- Mullard A. 2012 FDA drug approvals. Nat Rev Drug Discov. 2013;12(2):87–90. - PubMed
-
- Amur S, Frueh FW, Lesko LJ, et al. Integration and use of biomarkers in drug development, regulation and clinical practice: a US regulatory perspective. Biomark Med. 2008;2(3):305–11. - PubMed
-
- Wittchen HU, Jacobi F, Rehm J, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21(9):655–79. - PubMed
-
- Miller G. Is pharma running out of brainy ideas? Science. 2010;329(5991):502–4. - PubMed
-
- Biomarkers Definition Working group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

