Intersubunit communication in the dihydroorotase-aspartate transcarbamoylase complex of Aquifex aeolicus
- PMID: 24353170
- PMCID: PMC3892303
- DOI: 10.1002/pro.2396
Intersubunit communication in the dihydroorotase-aspartate transcarbamoylase complex of Aquifex aeolicus
Abstract
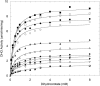
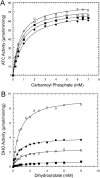
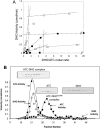
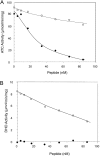
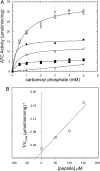
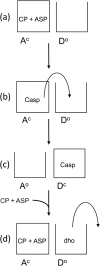
Aspartate transcarbamoylase and dihydroorotase, enzymes that catalyze the second and third step in de novo pyrimidine biosynthesis, are associated in dodecameric complexes in Aquifex aeolicus and many other organisms. The architecture of the dodecamer is ideally suited to channel the intermediate, carbamoyl aspartate from its site of synthesis on the ATC subunit to the active site of DHO, which catalyzes the next step in the pathway, because both reactions occur within a large, internal solvent-filled cavity. Channeling usually requires that the reactions of the enzymes are coordinated so that the rate of synthesis of the intermediate matches its rate of utilization. The linkage between the ATC and DHO subunits was demonstrated by showing that the binding of the bisubstrate analog, N-phosphonacetyl-L-aspartate to the ATC subunit inhibits the activity of the distal DHO subunit. Structural studies identified a DHO loop, loop A, interdigitating between the ATC domains that would be expected to interfere with domain closure essential for ATC catalysis. Mutation of the DHO residues in loop A that penetrate deeply between the two ATC domains inhibits the ATC activity by interfering with the normal reciprocal linkage between the two enzymes. Moreover, a synthetic peptide that mimics that part of the DHO loop that binds between the two ATC domains was found to be an allosteric or noncompletive ATC inhibitor (K(i) = 22 μM). A model is proposed suggesting that loop A is an important component of the functional linkage between the enzymes.
Keywords: CAD; N-phosphonacetyl-L-aspartate; allosteric regulation; aspartate transcarbamoylase; dihydroorotase; intersubunit communication; linkage; metabolic channeling; pyrimidine biosynthesis; thermophile.
© 2013 The Protein Society.
Figures

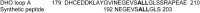
References
-
- Evans DR, Guy HI. Mammalian pyrimidine biosynthesis: fresh insights into an ancient pathway. J BIol Chem. 2004;279:33035–33038. - PubMed
-
- Deckert G, Warren PV, Gaasterland T, Young WG, Lenox AL, Graham DE, Overbeek R, Snead MA, Keller M, Aujay M, Huber R, Feldman RA, Short JM, Olsen GJ, Swanson RV. The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature. 1998;392:353–358. - PubMed
-
- Ahuja A, Purcarea C, Ebert R, Sadecki S, Guy HI, Evans DR. Aquifex aeolicus dihydroorotase: association with aspartate transcarbamoylase switches on catalytic activity. J Biol Chem. 2004;279:53136–53144. - PubMed
-
- Purcarea C, Ahuja A, Lu T, Kovari L, Guy HI, Evans DR. Aquifex aeolicus aspartate transcarbamoylase, an enzyme specialized for the efficient utilization of unstable carbamoyl phosphate at elevated temperature. J Biol Chem. 2003;278:52924–52934. - PubMed
-
- Zhang P, Martin PD, Purcarea C, Vaishnav A, Brunzelle JS, Fernando R, Guy-Evans HI, Evans DR, Edwards BF. Dihydroorotase from the hyperthermophile Aquifex aeolicus is activated by stoichiometric association with aspartate transcarbamoylase and forms a one-pot reactor for pyrimidine biosynthesis. Biochemistry. 2009;48:766–778. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

