Transcellular chaperone signaling: an organismal strategy for integrated cell stress responses
- PMID: 24353212
- PMCID: PMC3867495
- DOI: 10.1242/jeb.091249
Transcellular chaperone signaling: an organismal strategy for integrated cell stress responses
Abstract
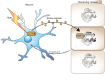
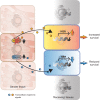
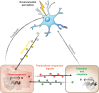
The ability of each cell within a metazoan to adapt to and survive environmental and physiological stress requires cellular stress-response mechanisms, such as the heat shock response (HSR). Recent advances reveal that cellular proteostasis and stress responses in metazoans are regulated by multiple layers of intercellular communication. This ensures that an imbalance of proteostasis that occurs within any single tissue 'at risk' is protected by a compensatory activation of a stress response in adjacent tissues that confers a community protective response. While each cell expresses the machinery for heat shock (HS) gene expression, the HSR is regulated cell non-autonomously in multicellular organisms, by neuronal signaling to the somatic tissues, and by transcellular chaperone signaling between somatic tissues and from somatic tissues to neurons. These cell non-autonomous processes ensure that the organismal HSR is orchestrated across multiple tissues and that transmission of stress signals between tissues can also override the neuronal control to reset cell- and tissue-specific proteostasis. Here, we discuss emerging concepts and insights into the complex cell non-autonomous mechanisms that control stress responses in metazoans and highlight the importance of intercellular communication for proteostasis maintenance in multicellular organisms.
Keywords: Caenorhabditis elegans; Cell non-autonomous; Chaperones; Metazoans; Proteostasis; Stress response.
Figures
Similar articles
-
Organismal proteostasis: role of cell-nonautonomous regulation and transcellular chaperone signaling.Genes Dev. 2014 Jul 15;28(14):1533-43. doi: 10.1101/gad.241125.114. Genes Dev. 2014. PMID: 25030693 Free PMC article. Review.
-
The mitochondrial unfolded protein response, a conserved stress response pathway with implications in health and disease.J Exp Biol. 2014 Jan 1;217(Pt 1):137-43. doi: 10.1242/jeb.090738. J Exp Biol. 2014. PMID: 24353213 Free PMC article. Review.
-
Integrating the stress response: lessons for neurodegenerative diseases from C. elegans.Trends Cell Biol. 2009 Feb;19(2):52-61. doi: 10.1016/j.tcb.2008.11.002. Epub 2008 Dec 26. Trends Cell Biol. 2009. PMID: 19112021 Free PMC article. Review.
-
Regulation of organismal proteostasis by transcellular chaperone signaling.Cell. 2013 Jun 6;153(6):1366-78. doi: 10.1016/j.cell.2013.05.015. Cell. 2013. PMID: 23746847 Free PMC article.
-
A neuronal GPCR is critical for the induction of the heat shock response in the nematode C. elegans.J Neurosci. 2013 Apr 3;33(14):6102-11. doi: 10.1523/JNEUROSCI.4023-12.2013. J Neurosci. 2013. PMID: 23554491 Free PMC article.
Cited by
-
A quantitative genome-wide RNAi screen in C. elegans for antifungal innate immunity genes.BMC Biol. 2016 Apr 29;14:35. doi: 10.1186/s12915-016-0256-3. BMC Biol. 2016. PMID: 27129311 Free PMC article.
-
Aberrant redox signalling and stress response in age-related muscle decline: Role in inter- and intra-cellular signalling.Free Radic Biol Med. 2019 Feb 20;132:50-57. doi: 10.1016/j.freeradbiomed.2018.11.038. Epub 2018 Nov 30. Free Radic Biol Med. 2019. PMID: 30508577 Free PMC article. Review.
-
Mahogunin Ring Finger-1 (MGRN1), a Multifaceted Ubiquitin Ligase: Recent Unraveling of Neurobiological Mechanisms.Mol Neurobiol. 2016 Sep;53(7):4484-96. doi: 10.1007/s12035-015-9379-8. Epub 2015 Aug 9. Mol Neurobiol. 2016. PMID: 26255182 Review.
-
Heat shock response during the resolution of inflammation and its progressive suppression in chronic-degenerative inflammatory diseases.Cell Stress Chaperones. 2024 Feb;29(1):116-142. doi: 10.1016/j.cstres.2024.01.002. Epub 2024 Jan 19. Cell Stress Chaperones. 2024. PMID: 38244765 Free PMC article. Review.
-
Dynamic O-linked N-acetylglucosamine modification of proteins affects stress responses and survival of mesothelial cells exposed to peritoneal dialysis fluids.J Am Soc Nephrol. 2014 Dec;25(12):2778-88. doi: 10.1681/ASN.2013101128. Epub 2014 May 22. J Am Soc Nephrol. 2014. PMID: 24854264 Free PMC article.
References
-
- Abravaya K., Phillips B., Morimoto R. I. (1991). Attenuation of the heat shock response in HeLa cells is mediated by the release of bound heat shock transcription factor and is modulated by changes in growth and in heat shock temperatures. Genes Dev. 5, 2117-2127 - PubMed
-
- Abravaya K., Myers M. P., Murphy S. P., Morimoto R. I. (1992). The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes Dev. 6, 1153-1164 - PubMed
-
- Alcedo J., Kenyon C. (2004). Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron 41, 45-55 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

