The mitochondrial unfolded protein response, a conserved stress response pathway with implications in health and disease
- PMID: 24353213
- PMCID: PMC3867496
- DOI: 10.1242/jeb.090738
The mitochondrial unfolded protein response, a conserved stress response pathway with implications in health and disease
Abstract
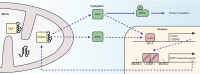
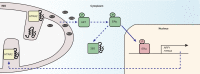
The ability to respond to various intracellular and/or extracellular stresses allows the organism to adapt to changing environmental conditions and drives evolution. It is now well accepted that a progressive decline of the efficiency of stress response pathways occurs with aging. In this context, a correct proteostasis is essential for the functionality of the cell, and its dysfunction has been associated with protein aggregation and age-related degenerative diseases. Complex response mechanisms have evolved to deal with unfolded protein stress in different subcellular compartments and their moderate activation translates into positive effects on health. In this review, we focus on the mitochondrial unfolded protein response (UPR(mt)), a response to proteotoxic stress specifically in mitochondria, an organelle with a wide array of fundamental functions, most notably the harvesting of energy from food and the control of cell death. We compare UPR(mt) with the extensively characterized cytosolic heat shock response (HSR) and the unfolded protein response in endoplasmic reticulum (UPR(ER)), and discuss the current knowledge about UPR(mt) signaling pathways as well as their potential involvement in physiology.
Keywords: Aging; Chaperones; Mitochondria; Proteostasis; Signaling.
Figures

References
-
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F., et al. (1981). Sequence and organization of the human mitochondrial genome. Nature 290, 457-465 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

