Stress adaptation in a pathogenic fungus
- PMID: 24353214
- PMCID: PMC3867497
- DOI: 10.1242/jeb.088930
Stress adaptation in a pathogenic fungus
Abstract
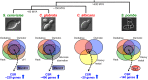
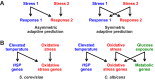
Candida albicans is a major fungal pathogen of humans. This yeast is carried by many individuals as a harmless commensal, but when immune defences are perturbed it causes mucosal infections (thrush). Additionally, when the immune system becomes severely compromised, C. albicans often causes life-threatening systemic infections. A battery of virulence factors and fitness attributes promote the pathogenicity of C. albicans. Fitness attributes include robust responses to local environmental stresses, the inactivation of which attenuates virulence. Stress signalling pathways in C. albicans include evolutionarily conserved modules. However, there has been rewiring of some stress regulatory circuitry such that the roles of a number of regulators in C. albicans have diverged relative to the benign model yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe. This reflects the specific evolution of C. albicans as an opportunistic pathogen obligately associated with warm-blooded animals, compared with other yeasts that are found across diverse environmental niches. Our understanding of C. albicans stress signalling is based primarily on the in vitro responses of glucose-grown cells to individual stresses. However, in vivo this pathogen occupies complex and dynamic host niches characterised by alternative carbon sources and simultaneous exposure to combinations of stresses (rather than individual stresses). It has become apparent that changes in carbon source strongly influence stress resistance, and that some combinatorial stresses exert non-additive effects upon C. albicans. These effects, which are relevant to fungus-host interactions during disease progression, are mediated by multiple mechanisms that include signalling and chemical crosstalk, stress pathway interference and a biological transistor.
Keywords: Candida albicans; Carbon metabolism; Cationic stress; Fungal pathogenicity; Heat shock; Nitrosative stress; Osmotic stress; Oxidative stress; Stress adaptation.
Figures




References
-
- Almeida R. S., Wilson D., Hube B. (2009). Candida albicans iron acquisition within the host. FEMS Yeast Res. 9, 1000-1012 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/C510391/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F00513X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 097377/WT_/Wellcome Trust/United Kingdom
- BB/D009308/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F010826/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

