Contrast-enhanced microCT (EPIC-μCT) ex vivo applied to the mouse and human jaw joint
- PMID: 24353248
- PMCID: PMC4064618
- DOI: 10.1259/dmfr.20130098
Contrast-enhanced microCT (EPIC-μCT) ex vivo applied to the mouse and human jaw joint
Abstract
Objectives: The temporomandibular joint (TMJ) is susceptive to the development of osteoarthritis (OA). More detailed knowledge of its development is essential to improve our insight into TMJ-OA. It is imperative to have a standardized reliable three-dimensional (3D) imaging method that allows for detailed assessment of both bone and cartilage in healthy and diseased joints. We aimed to determine the applicability of a contrast-enhanced microCT (µCT) technique for ex vivo research of mouse and human TMJs.
Methods: Equilibrium partitioning of an ionic contrast agent via µCT (EPIC-µCT) was previously applied for cartilage assessment in the knee joint. The method was ex vivo, applied to the mouse TMJ and adapted for the human TMJ.
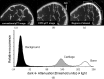
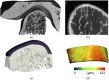
Results: EPIC-µCT (30-min immersion time) was applied to mouse mandibular condyles, and 3D imaging revealed an average cartilage thickness of 110 ± 16 µm. These measurements via EPIC-µCT were similar to the histomorphometric measures (113 ± 19 µm). For human healthy OA-affected TMJ samples, the protocol was adjusted to an immersion time of 1 h. 3D imaging revealed a significant thicker cartilage layer in joints with early signs of OA compared with healthy joints (414.2 ± 122.6 and 239.7 ± 50.5 µm, respectively). A subsequent significant thinner layer was found in human joints with late signs of OA (197.4 ± 159.7 µm).
Conclusions: The EPIC-µCT technique is effective for the ex vivo assessment of 3D cartilage morphology in the mouse as well as human TMJ and allows bone-cartilage interaction research in TMJ-OA.
Keywords: Hexabrix; articular cartilage; osteoarthritis; temporomandibular joint.
Figures




References
-
- Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res 1986; 213: 34–40. - PubMed
-
- Day JS, Van der Linden JC, Bank RA, Ding M, Hvid I, Summer DR, et al. . Adaptation of subchondral bone in osteoarthritis. Biorheology 2004; 41: 359–68. - PubMed
-
- Imhof H, Sulzbacher I, Grampp S, Czerny C, Youssefzadeh S, Kainberger F. Subchondral bone and cartilage disease: a rediscovered functional unit. Invest Radiol 2000; 35: 581–8. - PubMed
-
- Lajeunesse D, Reboul P. Subchondral bone in osteoarthritis: a biological link with articular cartilage leading to abnormal remodeling. Curr Opin Rheumatol 2003; 15: 628–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

